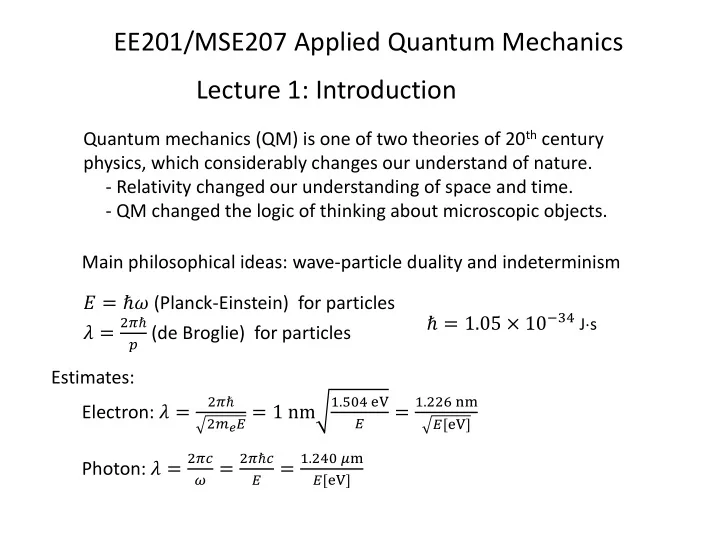

EE201/MSE207 Applied Quantum Mechanics Lecture 1: Introduction Quantum mechanics (QM) is one of two theories of 20 th century physics, which considerably changes our understand of nature. - Relativity changed our understanding of space and time. - QM changed the logic of thinking about microscopic objects. Main philosophical ideas: wave-particle duality and indeterminism 𝐹 = ℏ𝜕 (Planck-Einstein) for particles ℏ = 1.05 × 10 −34 J s 2𝜌ℏ 𝜇 = 𝑞 (de Broglie) for particles Estimates: 2𝜌ℏ 1.504 eV 1.226 nm Electron: 𝜇 = 2𝑛 𝑓 𝐹 = 1 nm = 𝐹 𝐹[eV] 2𝜌𝑑 2𝜌ℏ𝑑 1.240 𝜈m Photon: 𝜇 = 𝜕 = = 𝐹 𝐹[eV]
Prehistory of Quantum Mechanics 1) 1900 Max Planck: suggested discrete absorption and emission of light to explain experimental formula for black-body radiation (Nobel Prize 1918) 𝐹 = ℎ𝜉 = ℏ𝜕 ℎ = 6.63 × 10 −34 J s ℏ = 1.05 × 10 −34 J s 2) 1905 Albert Einstein: theory of photoelectric effect (Nobel Prize 1921) 𝑛𝑤 2 ℎ𝜉 = Φ + Φ is work function (ionization energy) 2 3) 1913 Niels Bohr: model of atom (Nobel Prize 1922) 𝑛𝑤𝑠 = 𝑜ℏ Discrete atomic spectra and Rutherford’s experiments (1910 -1911, N.P. 1908) 4) 1923 Arthur Compton: scattering of X-rays on electrons (Nobel Prize 1927) 5) 1923 Louis de Broglie: matter waves (theory only, in 1927 confirmed for 𝜇 = 2𝜌ℏ electrons, Nobel Prize 1929) 𝑞 “Birth” of Quantum mechanics: 1927 (5 th Solvay conference, Brussels)
Classical mechanics vs. Quantum Mechanics (one particle in one dimension) Classical mechanics: 𝑒 2 𝑦 𝑒𝑦 Position 𝑦(𝑢) , velocity 𝑤 𝑢 = 𝑒𝑢 , acceleration 𝑏 𝑢 = 𝑒𝑢 2 𝑒𝑊(𝑦) 𝐺 = − for a conservative system, 𝑊(𝑦) is potential energy 𝑒𝑦 𝐺 Main evolution equation: 𝑏 = 𝑛 𝑒 2 𝑦 𝑒𝑢 2 = − 1 𝑒𝑊 initial conditions: 𝑦(𝑢) 𝑦 0 , 𝑦(0) 𝑛 𝑒𝑦 Quantum mechanics: Main evolution equation: Schrödinger equation 𝜖𝑢 = − ℏ 2 𝜖 2 Ψ 𝑗ℏ 𝜖Ψ Ψ(𝑦, 𝑢) is a complex function, characterizing 𝜖𝑦 2 + 𝑊Ψ 2𝑛 the particle state (wave function) 2 𝑒𝑦 is the probability to find the particle between 𝑦 and 𝑦 + 𝑒𝑦 Ψ 𝑦, 𝑢 at time 𝑢 (if observed!) Indeterminacy: particle does not have position (still debates about philosophy)
Recommend
More recommend