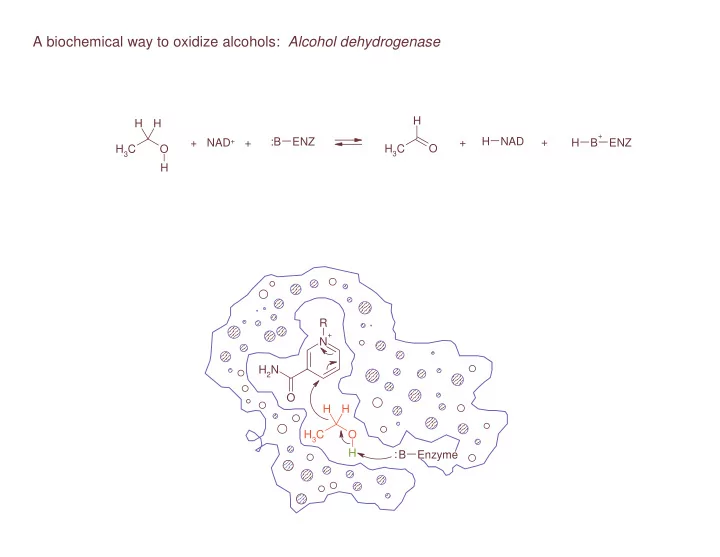

A biochemical way to oxidize alcohols: Alcohol dehydrogenase H H H + ENZ :B ENZ H NAD + NAD + + + + H B H 3 C O H 3 C O H R + N H 2 N O H H H 3 C O H : B Enzyme
The enzyme, :B–ENZ, has a basic functional group in proximity to where ethanol will bind : B Enzyme
There also is a binding site for NAD + R + N H 2 N O : B Enzyme
When the alcohol binds in the acitve site of the enzyme, an elimination reaction can proceed R + N H 2 N O H H H 3 C O H : B Enzyme
Deprtonation by the base R + N H 2 N O H H H 3 C O H : B Enzyme
allows for C=O double bond formation R + N H 2 N O H H H 3 C O H : B Enzyme
the formal leaving group is H - , a hydride, which can be added to NAD + R + N H 2 N O H H H 3 C O H : B Enzyme
with allylic rearrangement of the pi electon system R + N H 2 N O H H H 3 C O H : B Enzyme
to quench the formal positive change of nitrogen R + N H 2 N O H H H 3 C O H : B Enzyme . . .It’s an E 2 -type elimination reaction!
H H H + ENZ :B ENZ H NAD + NAD + + + + H B H 3 C O H 3 C O H R N .. H 2 N H O H H 3 C O + Enzyme H B
Recommend
More recommend