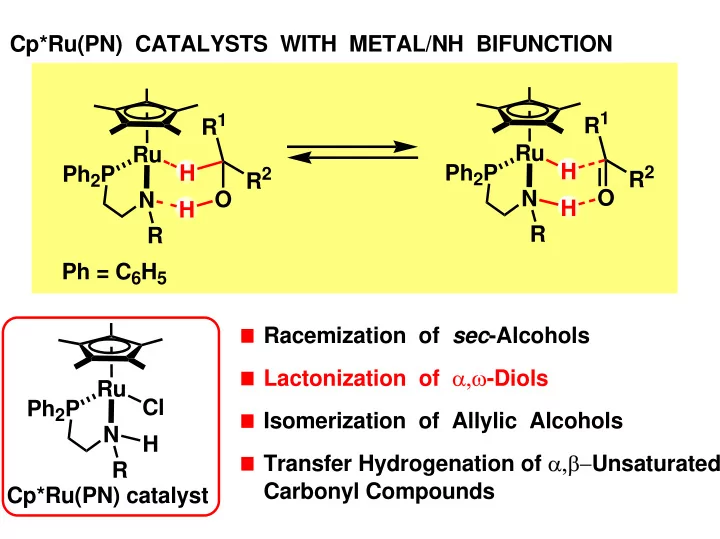

Cp*Ru(PN) CATALYSTS WITH METAL/NH BIFUNCTION R 1 R 1 Ru Ru H H Ph 2 P Ph 2 P R 2 R 2 O O N N H H R R Ph = C 6 H 5 Racemization of sec -Alcohols Lactonization of α,ω -Diols Ru Cl Ph 2 P Isomerization of Allylic Alcohols N H Transfer Hydrogenation of α,β− Unsaturated R Carbonyl Compounds Cp*Ru(PN) catalyst
LACTONIZATION OF SYMMETRICAL 1,4-DIOL O Ru cat R R O OH OH KO t -C 4 H 9 + OH + 2 2 O acetone R R 30 °C, 1 h TOF >1000 h − 1 >99% yield diol:Ru cat:KO t -C 4 H 9 = 100:1:1, [diol] = 0.1 ~ 1.0 M H [Ru] R R OH O OH OH R R Ph 2 [Ru]-H 2 P Ru N Cl O HO [Ru] H 2 H R R Ru cat O O R R [Ru]-H 2 [Ru] = Cp*Ru(PN) The 81st Annual Meeting of Chemical Society of Japan . 2002 , 3G414
LACTONIZATION OF UNSYMMETRICAL 1,4-DIOL R 1 R 2 R 1 R 2 Cp*RuCl(PN) O OH KO t -C 4 H 9 OH + 2 O 2 + OH acetone 30 °C, 1 h O PN = Ph 2 P(CH 2 ) 2 NH 2 diol:Ru cat:KO t -C 4 H 9 = 100:1:1, [diol] = 0.5 M R 1 R 2 yield, % CH 3 H >99 CH 3 D (96%) >99 (96% atom D) CH 3 CH 3 >99 The 79th Annual Meeting of Chemical Society of Japan . 2001 , 1H326 THIS WORK Regioselective lactonization of 2-substituted 1,4-butanediols O Ru cat 1 R R R 1 1 O OH KO t -C 4 H 9 OH 2 2 2 + 2 + + 2 O O OH acetone 4 4 4 O
REPORTED LACTONIZATION OF UNSYMMETRICAL DIOLS O O O R R R Ru cat R' R' R' CH 3 OH CH 3 + 2 + + 2 O O toluene OH Ph Ph 20 °C, 10 h O diol:Ru cat = 25:1 A B yield, % A:B RuH 2 (PPh 3 ) 4 R = CH 3 ; R' = CH 3 100 99.6:0.4 R = i - (C 3 H 7 ); R' = H 100 98:2 Ru cat R = Ph; R' = H 90 97:3 Ishii, Y.; Osakada, K.; Ikariya, T.; Saburi, M.; Yoshikawa, S. J. Org. Chem . 1986 , 51 , 2034 O Ph Ph Ph Ph Ph Ir cat Ph OH + O O OH acetone rt, 20 h O diol:Ir cat = 200:1 98% yield A B Ir HN O A:B = >99:1 Ph Ph Suzuki, T.; Morita, K.; Tsutida, M.; Hiroi, K. Org. Lett. 2002, 4, 2361 Ir cat
LIGAND EFFECT:Cp STRUCTURE O Ru cat Ph Ph KO t -C 4 H 9 Ph OH + O O OH acetone 30 °C, 1 h O Ph = C 6 H 5 diol:Ru cat:KO t -C 4 H 9 = 100:1:1, [diol] = 0.5 M A B Ru cat yield, % A:B* PF 6 Ph 2 P Ru >99 56:44 N NCCH 3 H 2 OTf Ph 2 P Ru >99 78:22 N NCCH 3 H 2 * Determined by 1 H NMR analysis
LIGAND EFFECT: N- & P- & N- α -SUBSTITUENTS P N A:B P N A:B OTf Ph P Ru 78:22 81:19 NH 2 Ph 2 P N NH 2 Ph 2 P NCCH 3 CH 2 Ph Ru cat 82:18 76:24 NHCH 3 Ph 2 P NH 2 Ph 2 P CH 2 Ph 4-Tol 83:17 78:22 NHCH 2 Ph NH 2 Ph 2 P (4-Tol) 2 P CH 2 Ph 2,6-Xy 81:19 (2,6-Xy) 2 NH 2 P
LIGAND EFFECT: BACKBONE O Ru cat Ph Ph KO t -C 4 H 9 Ph OH + O O OH acetone 30 °C, 1 h O diol:Ru cat:KO t -C 4 H 9 = 100:1:1, [diol] = 0.5 M A B yield, % A:B P N >99 82:18 Ph 2 P NH 2 OTf >99 92:8 P Ph 2 P NH 2 Ru N NCCH 3 47 92:8 Ru cat Ph 2 P NCH 3 H 20 92:8 Ph 2 P NCH 2 Ph H
EFFECT OF ARYLMETHYL SUBSTITUENTS IN 1,4-DIOL O Ru cat Ar Ar KO t -C 4 H 9 Ar OH + O O OH acetone 30 °C, 1 h O >99% yield A B diol:Ru cat:KO t -C 4 H 9 = 100:1:1, [diol] = 0.5 M Ar A:B 92:8 OTf Ph 2 O P 92:8 Ru O N NCCH 3 H 2 92:8 CH 3 O Ru cat 92:8 PhCH 2 O
BIOLOGICALLY IMPORTANT LIGNANS HO O O O O O O HO O O ( − )-Enterolactone O R n ( − )-Hinokinin O CH 3 O O O OH HO O O OH O O O O O O O O O O CH 3 O O CH 3 O OCH 3 CH 3 O CH 3 O OCH 3 OCH 3 OCH 3 OCH 3 ( − )-Podophyllotoxin (+)-Isostegane Etoposide
SYNTHESIS OF JUSTICIDIN E O O O O Ru cat OH O O KO t -C 4 H 9 OH O O O + acetone O 30 °C, 15 h O O O O O O Justicidin E Taiwanin C diol:Ru cat:KO t -C 4 H 9 =50:1:1 94% 0% OTf Ph 2 P Ru N NCCH 3 H 2 Ru cat
SUMMARY O Ru cat Ar KO t -C 4 H 9 Ar Ar OH + O O acetone OH 30 °C, 1h O 92:8 diol:Ru cat:KO t -C 4 H 9 = 100:1:1, [diol] = 0.5 M OTf O Ph 2 P Ar = O Ru N NCCH 3 H 2 CH 3 O Ru cat PhCH 2 O Regiocontrol by Cp ligand structure and PN chelate ring size
Recommend
More recommend