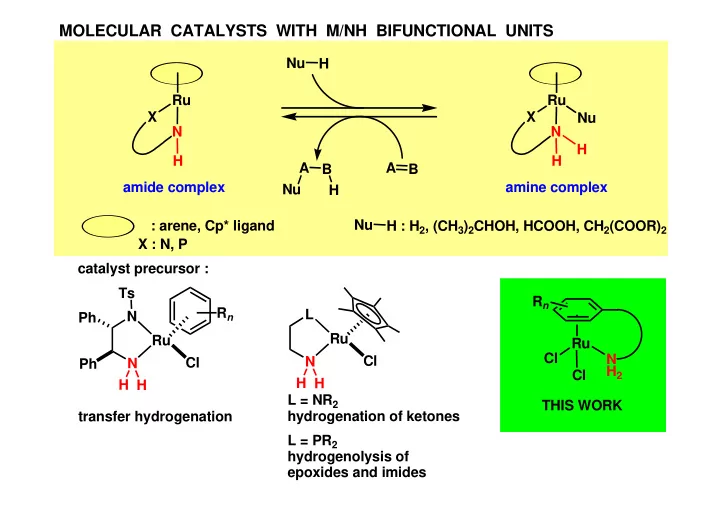

MOLECULAR CATALYSTS WITH M/NH BIFUNCTIONAL UNITS Nu H Ru Ru X X Nu N N H H H A A B B amide complex amine complex Nu H Nu : arene, Cp* ligand H : H 2 , (CH 3 ) 2 CHOH, HCOOH, CH 2 (COOR) 2 X : N, P catalyst precursor : Ts R n R n L N Ph Ru Ru Ru Cl N Cl N Cl N Ph H 2 Cl H H H H L = NR 2 THIS WORK transfer hydrogenation hydrogenation of ketones L = PR 2 hydrogenolysis of epoxides and imides
REPORTED EXAMPLES OF η 1 : η 6 -(AMINOARENE)Ru COMPLEXES η 6 − arene → η 1 : η 6 − L − arene NH 2 NH 3 Cl 1) Birch reduction n-1 n-1 2) HCl (CH 2 ) n NH 3 Cl BF 4 RuCl 3 x H 2 O Cl Ru Ru EtOH Ph 3 P n − 2 N Cl 2 reflux Cl H 2 n = 2, 3 Kurosawa , Inorg. Chim. Acta , 2000 Ph Ph HN Ru SO 2 SO 2 NH 3 Cl N(C 2 H 5 ) 3 Cl N H 2 N 2-propanol Cl reflux Ph Ph Ru Cl 2 Wills , J. Am. Chem. Soc. , 2004
THIS WORK : INTRAMOLECULAR ARENE DISPLACEMENT REACTION η 1 − L → η 1 : η 6 − L − arene CO 2 Et Cl + NH 2 Ru Cl 2 EtOCO ∆ Ru Ru Cl Cl N NH 2 H 2 Cl Cl η 1 :η 6 − tethered complex η 1 − NH 2 complex
EFFECT OF SUBSTITUENTS FOR η 1 : η 6 -(AMINOARENE)Ru COMPLEXES EtOCO R R Ru chlorobenzene Ru Cl Cl N N 140 °C H 2 H 2 Cl Cl R time, h yield, % a H 21 >99 m -CH 3 18 >99 p -CH 3 20 62 m,p -(CH 3 ) 2 17 67 o -Si(CH 3 ) 3 2 80 m -Si(CH 3 ) 3 2 88 p -Si(CH 3 ) 3 2 90 [Ru] = 0.02 M. a Isolated yield.
EFFECT OF STRUCTURE OF AMINOARENES NH 2 NH 2 R R 1 R 2 R 1 R 2 R time, h time, h yield, % a yield, % a CH 3 CH 3 18 98 H 16 94 m -Si(CH 3 ) 3 2 CO 2 CH 3 H 13 98 69 p -Si(CH 3 ) 3 2 56 [Ru] = 0.02 M. a Isolated yield. O Y NH 2 R R time, h Y yield, % a p -Si(CH 3 ) 3 CH 2 2 <1 p -Si(CH 3 ) 3 CO 1 <1
SPECTRAL DATA (CH 3 ) 3 Si ESI-TOF Ru Cl N H 2 Cl 1 H NMR C H Cl 3 TMS η 6 -C 6 H 4 Si(C H 3 ) 3 + (CH 3 ) 3 Si Ru N Cl H 2
SYNTHESIS OF CATIONIC ARENE − Ru COMPLEXES (CH 2 ) 2 NH 3 Cl + NaOH + NaBF 4 Ru + PPh 3 + AgBF 4 Ru N Cl Ph 3 P H 2 Cl Cl Cl methanol methanol reflux, 20 h rt, 15 h 56% yield 42% yield Kurosawa , Inorg. Chim. Acta , 2000 this work BF 4 Ru N Ph 3 P H 2 Cl
STEREOSELECTIVE FORMATION OF PHOSPHINE COMPLEXES phosphine complex with ethylene tether (CH 3 ) 3 Si (CH 3 ) 3 Si SbF 6 PPh 3 + AgSbF 6 methanol Ru Ru reflux, 18 h N N Cl Cl H 2 H 2 Cl PPh 3 anti 70% yield single diastereomer ( 31 P NMR) Selected bond lengths[Å] and angles[°] Ru − C(1) 2.152(9) Ru − C(2) 2.221(9) Ru − C(3) 2.276(8) Ru − C(4) 2.241(7) Ru − C(5) 2.197(6) Ru − C(6) 2.174(7) Ru − Cl 2.404(2) Ru − N 2.157(7) Ru − P 2.360(2) P − Ru − Cl 87.90(7) P − Ru − N 92.0(2) N − Ru − Cl 84.8(2) R 1 ( I > 2 σ ) = 0.094 w R 2 = 0.175 P -1 (#2) Z = 2
phosphine complex with propylene tether (CH 3 ) 3 Si (CH 3 ) 3 Si SbF 6 PPh 3 + AgSbF 6 Ru CH 2 Cl 2 Ru Cl N rt, 24 h N Cl H 2 Cl H 2 PPh 3 anti 52% yield single diastereomer ( 31 P NMR) Selected bond lengths[Å] and angles[°] Ru − C(1) 2.211(4) Ru − C(2) 2.255(4) Ru − C(3) 2.267(5) Ru − C(4) 2.217(5) Ru − C(5) 2.186(6) Ru − C(6) 2.222(6) Ru − Cl 2.3969(13) Ru − N 2.135(4) Ru − P 2.3541(12) P − Ru − Cl 86.13(4) P − Ru − N 90.93(13) N − Ru − Cl 81.05(13) R 1 ( I > 2 σ ) = 0.053 w R 2 = 0.143 P -1 (#2) Z = 4
SYNTHESIS OF CATIONIC ISOCYANIDE COMPLEX SbF 6 (CH 3 ) 3 Si (CH 3 ) 3 Si AgSbF 6 t -C 4 H 9 NC + + Ru Ru N N CH 2 Cl 2 , rt Cl Cl H 2 H 2 2 h, 88% yield Cl CN t -C 4 H 9 IR ν N ≡ C 2201 cm -1 SbF 6 (CH 3 ) 3 Si (CH 3 ) 3 Si AgSbF 6 t -C 4 H 9 NC + + Ru Ru CH 2 Cl 2 , rt Cl Cl N N 2 h, 49% yield H 2 H 2 Cl CNt-C 4 H 9 IR ν N ≡ C 2197 cm -1
isocyanide complex with propylene tether (CH 3 ) 3 Si (CH 3 ) 3 Si (CH 3 ) 3 Si SbF 6 SbF 6 t -C 4 H 9 NC AgSbF 6 + Ru CH 2 Cl 2 Ru Ru N rt, 1 h N Cl N Cl t -C 4 H 9 NC H 2 H 2 H 2 Cl Cl CN t -C 4 H 9 syn 16% yield anti IR ν N ≡ C 2193 cm -1 Selected bond lengths[Å] and angles[°] Ru − Cl 2.3911(13) Ru − N(1) 2.142(3) Ru − C(13) 1.989(4) N(2) − C(13) 1.142(5) C(13) − Ru − Cl 83.34(13) C(13) − Ru − N 86.91(12) R 1 ( I > 2 σ ) = 0.044 N − Ru − Cl 81.62(11) w R 2 = 0.090 P -1 (#2) Z = 2
EFFECT OF AMINO-TETHER IN HYDROGEN TRANSFER O OH Ru cat t -C 4 H 9 OK H 2-propanol 30 °C, 1 h ketone:Ru: t -C 4 H 9 OK = 10:1:1, [ketone] = 0.1 M (CH 3 ) 3 Si (CH 3 ) 3 Si (CH 3 ) 3 Si Ru Ru Ru Cl N Cl N Cl N Cl H 2 Cl H 2 Cl H 2 yield, % a 28 >99 <1 (without base) Si(CH 3 ) 3 (CH 3 ) 3 Si Cl Ru Ru NR 1 R 1 = n -C 6 H 13 Cl Cl 2 Cl H 2 yield, % a 7 9 a Determined by 1 H NMR.
HYDROGEN TRANSFER USING CATIONIC COMPLEXES O OH Ru cat t -C 4 H 9 OK H 2-propanol 30 °C, 1 h ketone:Ru: t -C 4 H 9 OK = 10:1:1, [ketone] = 0.1 M SbF 6 SbF 6 (CH 3 ) 3 Si (CH 3 ) 3 Si (CH 3 ) 3 Si Ru Ru Ru Cl N N N Cl Cl Cl H 2 H 2 H 2 PPh 3 CN t -C 4 H 9 yield, % a >99 10 <1 a Determined by 1 H NMR.
PROPOSED MECHANISM SbF 6 base Ru Ru – HSbF 6 Cl Cl N N H 2 H L L L – L base Ru Ru Ru – HCl Cl N Cl N Cl N H 2 H 2 Cl H H + + OH O R R H
SUMMARY EtOCO ∆ Ru Ru Cl NH 2 Cl N Cl H 2 Cl η 1 − NH 2 complex η 1 :η 6 − tethered complex Novel synthetic method for various η 1 :η 6 -(aminoarene)Ru complexes Stereocontrol based on a planar chirality Potent hydrogen transfer ability
Recommend
More recommend