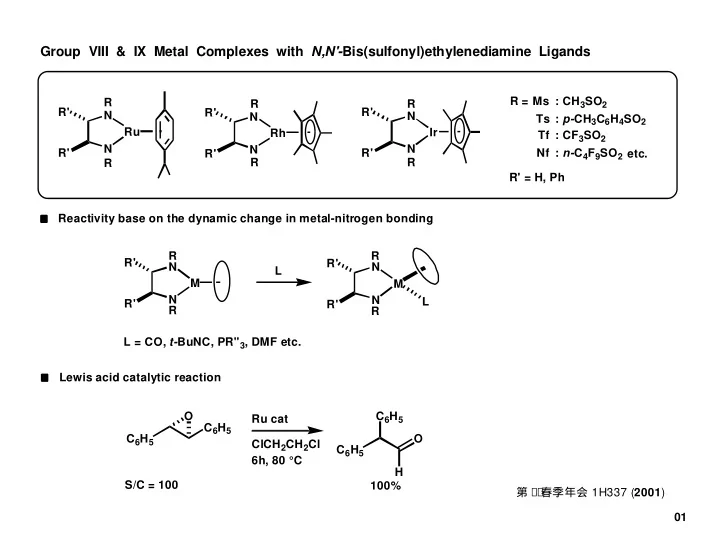

Group VIII & IX Metal Complexes with N,N'- Bis(sulfonyl)ethylenediamine Ligands R = Ms : CH 3 SO 2 R R R R' R' R' N N N Ts : p- CH 3 C 6 H 4 SO 2 Ru Ir Rh Tf : CF 3 SO 2 N N N R' R' Nf : n- C 4 F 9 SO 2 R' etc. R R R R' = H, Ph Reactivity base on the dynamic change in metal-nitrogen bonding R R R' R' N N L M M N N L R' R' R R L = CO, t- BuNC, PR'' 3 , DMF etc. Lewis acid catalytic reaction O C 6 H 5 Ru cat C 6 H 5 C 6 H 5 O ClCH 2 CH 2 Cl C 6 H 5 6h, 80 °C H S/C = 100 100% 第7 9 春季年会 1H337 ( 2001 ) 01
New N,N'- bis(sulfonylamido)Complexes with Multiple Reaction Sites Half-sandwich type complexes with arene or Cp* ligand R R SO 2 SO 2 N N Ru + Ru N N SO 2 substrate SO 2 R R single reaction site This work Ru or Pd complexes with labile ligands L 4 Ru complex L 2 Pd complex R R SO 2 SO 2 L N N L L multiple reaction site Ru Pd L L N N L SO 2 SO 2 R R 02
Preparation of L 4 Ru Complexes with N,N'- bis(sulfonyl)ethylenediamine ( t- BuNC) 4 Ru complex Tf CN t- Bu Tf C 6 H 5 t- BuNC (20 equiv.) N C 6 H 5 N CN t- Bu Ru Ru THF, reflux N C 6 H 5 N CN t- Bu C 6 H 5 12 h Tf Tf CN t- Bu 84% [starting Ru complex] = 1.0 x 10 -1 M (CH 3 CN) 4 Ru complex NCCH 3 Tf Tf C 6 H 5 N C 6 H 5 N NCCH 3 h ν (Hg lamp) Ru Ru CH 3 CN, rt N C 6 H 5 N NCCH 3 C 6 H 5 Tf 1 day Tf NCCH 3 98% [starting Ru complex] = 1.0 x 10 -2 M 03
Ru[( S,S )-Tf 2 dpen]( t- BuNC) 4 Ru[( S,S )-Tf 2 dpen](CH 3 CN) 4 N3 C27 N2 N1 C22 N1 Ru1 Ru1 N1* N2 C17 N2* C32 N3* P1 (#1) C2 (#5) R R 0.055 0.050 0.069 0.060 R w R w side view side view Selected bond Selected bond lengths(Å) lengths(Å) 168.8(3)° ca. 180° Ru1-C17 1.936(7) Ru1-N2 2.000(9) Ru1-C22 1.937(7) Ru1-N3 2.026(6) Ru1-C27 2.019(8) Ru1-C32 2.023(7) Ru1-N1 2.165(8) Ru1-N1 2.176(5) Ru1-N2 2.185(5) 04
Reaction of Ru[( S,S )-Tf 2 dpen](CH 3 CN) 4 with Various Ligands 1 H NMR ( δ /ppm) XylNC NCCH 3 CNXyl δ 2.29 ppm (s, 12H, CH 3 of XylNC) Tf Tf C 6 H 5 NCCH 3 (2.0 equiv.) C 6 H 5 N NCCH 3 N δ 2.36 ppm (s, 6H, C H 3 CN) Ru Ru CH 2 Cl 2 , rt δ 5.22 ppm (s, 2H, C H PhC H Ph) N N C 6 H 5 NCCH 3 C 6 H 5 NCCH 3 24 h Tf Tf 19 F NMR ( δ /ppm) NCCH 3 CNXyl δ -75.16 ppm (s, SO 2 C F 3 ) 95% IR (KBr): 2121 cm -1 XylNC = 2,6-(CH 3 ) 2 C 6 H 3 NC N N N N Tf Tf C 6 H 5 C 6 H 5 N N NCCH 3 N NCCH 3 Tf C 6 H 5 N NCCH 3 Ru or Ru (1.0 equiv.) N N Ru C 6 H 5 NCCH 3 C 6 H 5 N Tf Tf NCCH 3 NCCH 3 N ClCH 2 CH 2 Cl, 60°C C 6 H 5 NCCH 3 Tf NCCH 3 Λ ∆ 4 days 86% single diastereomer N N 1 H NMR (δ /ppm) N N = 1.95, 2.63 (bs, s, 3H, C H 3 CN) 5.11, 5.81 (s, 2H, N H PhN H Ph) 19 F NMR (δ /ppm) -77.17, -75.43 (C F 3 , each s) 05
Preparation of N,N'- bis(sulfonylamido)Pd(II) Complexes R R C 6 H 5 NH C 6 H 5 N NCCH 3 Pd(OAc) 2 + 2 AcOH Pd + CH 3 CN, reflux NCCH 3 N C 6 H 5 NH C 6 H 5 R R 24 h 1.0 equiv. pale yellow Ts Tf Nf Pf C 6 H 5 C 6 H 5 C 6 H 5 C 6 H 5 N N N NCCH 3 N NCCH 3 NCCH 3 NCCH 3 Pd Pd Pd Pd NCCH 3 N N NCCH 3 N NCCH 3 N NCCH 3 C 6 H 5 C 6 H 5 C 6 H 5 C 6 H 5 Ts Tf Nf Pf 32% 81% 80% 66% Nf = n- C 4 F 9 SO 2 - Pf = C 6 F 5 SO 2 - 06
Reaction of Pd Complex with Various Ligands Tf Tf C 6 H 5 C 6 H 5 N L N NCCH 3 L Pd Pd NCCH 3 L solvent, rt N N C 6 H 5 C 6 H 5 Tf Tf - 2CH 3 CN colorless pale yellow solvent L 2 equiv. yield, % t- BuNC 2.1 THF 99 N N CH 2 Cl 2 1.0 99 98 1.5 THF 1.5 THF a 96 Characterized by 1 H and 19 F NMR. a In the presence of MS 4A. 07
Rearrangement of Allyl Ester via Kinetic Resolution Pd cat O O O O O O O O + + ClCH 2 CH 2 Cl C 6 H 5 C 6 H 5 C 6 H 5 C 6 H 5 60 °C, 8 h S R R S/C = 20 Pd cat ee, % a k S /k R b conv, % a Pd[( S,S )-Tf 2 dpen](CH 3 CN) 2 62 43.7 2.5 Pd[( S,S )-Ts 2 dpen](CH 3 CN) 2 3 0.3 1.0 Pd[( S,S )-Nf 2 dpen](CH 3 CN) 2 48 48.0 2.1 Pd[( S,S )-Pf 2 dpen](CH 3 CN) 2 59 60.5 4.3 Conditions; [ester] = 2.0 x 10 -1 M. a Determined by GC (CHIRASIL DEX-CB) and n- dodecane as an internal standard. b Calculated based on the equation shown below. ln (1-conv)(1- ee) k S /k R = ln (1-conv)(1+ee) 08
SUMMARY Stereospecific Ligand Substitution R R R NCCH 3 SO 2 NCCH 3 L C 6 H 5 SO 2 SO 2 N NCCH 3 C 6 H 5 C 6 H 5 N N NCCH 3 L NCCH 3 Ru Ru Ru NCCH 3 N NCCH 3 NCCH 3 N N C 6 H 5 -CH 3 CN C 6 H 5 C 6 H 5 SO 2 NCCH 3 SO 2 NCCH 3 SO 2 L R R R Asymmetric [3,3]-Sigmatropic Rearrangement R SO 2 C 6 H 5 Pd cat N O O NCCH 3 O O O O O O + + Pd C 6 H 5 NCCH 3 N C 6 H 5 C 6 H 5 C 6 H 5 C 6 H 5 SO 2 R S R R k S / k R = up to 4.3 09
Recommend
More recommend