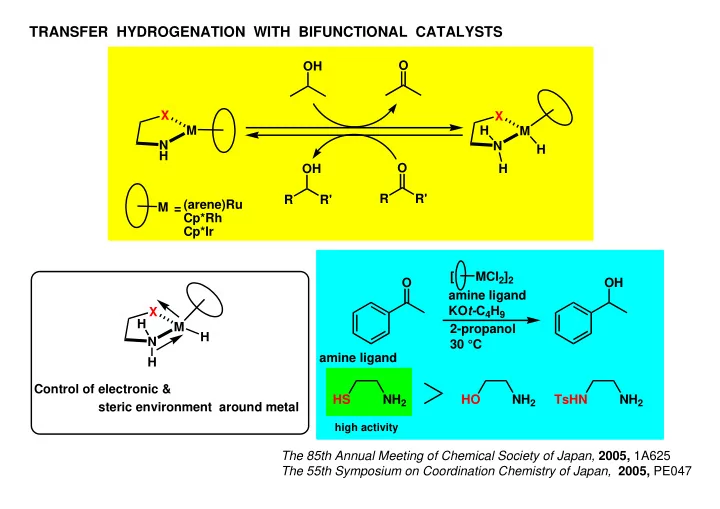

TRANSFER HYDROGENATION WITH BIFUNCTIONAL CATALYSTS O OH X X M H M N N H H O OH H R R' R R' (arene)Ru M = Cp*Rh Cp*Ir [ MCl 2 ] 2 O OH amine ligand KO t- C 4 H 9 X H M 2-propanol H N 30 °C amine ligand H Control of electronic & NH 2 NH 2 NH 2 HS HO TsHN steric environment around metal high activity The 85th Annual Meeting of Chemical Society of Japan, 2005, 1A625 The 55th Symposium on Coordination Chemistry of Japan, 2005, PE047
TRANSFER HYDROGENATION WITH 2-PROPANOL NH 3 (OTf) 2 H 2 N Cl 2 [RuCl 2 (hmb)] 2 H 2 N Cl S S Ru Ru + Ru Ru S S NH 2 HS NH 2 A B O OH OH O cat + + 30 °C, 1 h ketone:Ru = 100:1 [ketone] = 1 M in 2-propanol cat A + KO t- C 4 H 9 (3 equiv) B + KO t- C 4 H 9 (2 equiv) yield 81% 79% hmb = C 6 (CH 3 ) 6
A POSSIBLE ROUTE FOR GENERATION OF ACTIVE CATALYST NH 3 (OTf) 2 Cl 2 H 2 N Cl H 2 N S –HCl NH S –HOTf S Ru Ru Ru Ru Ru Ru base base S S S HN 3 equiv 2 equiv NH 2 18e OH O 16e Ru Ru S H NH S OH O NH 2
NH 3 THIS WORK Cl 2 H 2 N Cl S Ru Ru S O OH H 2 N SH achiral ligand O OH R NH 3 Ph H 2 N SH Cl 2 Ph H 2 N Cl chiral ligand S Ru Ru S
REPORTED SYNTHESIS OF N - α -SUBSTITUTED SN COMPOUNDS R CS 2 R Br S NH NH 2 S HBr R HO S NH 2 H 2 N NH 2 R R HS Br NH 2 NH 2 O O R R SH HS R HO S HN HN Boc HN Boc Boc T. P. Johnston, J. Med. Chem., 1966, 9, 911 H. H. Otto, Helv. Chim. Acta, 2004 , 87 , 90 Boc = t- C 4 H 9 OCO B. P. Roques, J. Med. Chem. , 1992 , 35, 1259
SYNTHESIS OF N - α -SUBSTITUTED SN LIGANDS R R KO t- C 4 H 9 SH + HN O t- C 4 H 9 OH reflux H 2 N S O 25% R = i- C 3 H 7 C 6 H 5 95% CH 2 C 6 H 5 82% R R 1) Na/NH 3 2) HCl/ether H 2 N S ClH 3 N SH R = i- C 3 H 7 50% C 6 H 5 73% CH 2 C 6 H 5 98% R R KO t- C 4 H 9 + C 6 H 5 SH HN O t- C 4 H 9 OH H 2 N SC 6 H 5 reflux O Ishibashi, H. Synlett, 1997, 915
SYNTHESIS OF CHIRAL THIOLATE–BRIDGED DIMER NH 3 Ph Cl 2 Ph H 2 N Cl NH 3 Cl KO t- C 4 H 9 S HS [RuCl 2 (hmb)] 2 + Ru Ru 2-propanol Ph S r.t. quantitative [RuCl 2 (hmb)] 2 :amine:base = 1:2:3 1 H NMR ( δ , CDCl 3 ): 1.95 (s, 18H, hmb) 2.14 (s, 18H, hmb) 5.63 (s, 1H, N H ) 6.05 (s, 1H, N H ) 8.68 (s, 3H, N H 3 ) ESI-MS: 867 NH 3 Ph Cl 2 Ph H 2 N Cl NH 3 Cl KO t- C 4 H 9 S HS [Cp*MCl 2 ] 2 + M M 2-propanol S Ph r.t. M = Rh, Ir quantitative Ph = C 6 H 5 [Cp*MCl 2 ] 2 :amine:base = 1:2:3
ASYMMETRIC TRANSFER HYDROGENATION WITH 2-PROPANOL metal cat metal cat O OH OH O KO t- C 4 H 9 NH 3 Ph + + Cl 2 S 30 °C Ph H 2 N Cl S M M ketone:M = 1000:1 S [ketone] = 1 M in 2-propanol 1 0 0 1 0 0 8 0 8 0 R u ( h m b ) e e C p * R h % % 6 0 6 0 / C p * I r / d l t e c R u ( h m b ) i 4 0 4 0 e y l e C p * R h s 2 0 C p * I r 2 0 0 0 0 6 1 2 1 8 2 4 0 6 1 2 1 8 2 4 r e a c t i o n t i m e / h r e a c t i o n t i m e / h
TRANSFER HYDROGENATION WITH FORMIC ACID metal cat O OH N(CH 2 CH 3 ) 3 + + CO 2 HCOOH S 30 °C ketone:M = 100:1 ketone:HCOOH:N(CH 2 CH 3 ) 3 = 2:3:5 metal cat : % ee (yield) at 24 h % ee (yield) at 1 h M NH 3 Cl 2 Ph Ru(hmb) 75 (19) 76 (81) Ph H 2 N Cl Cp*Rh 74 (22) 65 (46) S M M S Cp*Ir 58 (19) 52 (74)
SUMMARY R C 6 H 5 CH 2 SH R R KO t- C 4 H 9 1) Na/NH 3 HN O R = C 6 H 5 2) HCl i- C 3 H 7 t- C 4 H 9 OH H 2 N S ClH 3 N SH CH 2 C 6 H 5 O NH 3 Ph Ph Cl 2 KO t- C 4 H 9 Ph H 2 N Cl [ MCl 2 ] 2 + 2-propanol S ClH 3 N SH M M = Ru(hmb) r.t. M S Cp*Rh :amine:base = 1:2:3 Cp*Ir [ MCl 2 ] 2 O OH ( S,S )-Ru 2 (SN) 2 HCOOH/N(CH 2 CH 3 ) 3 S 30 °C 24 h 75% ee ketone:Ru = 100:1 ketone:HCOOH:N(CH 2 CH 3 ) 3 = 2:3:5
SYNTHESIS OF RuSN COMPLEX OTf [RuCl 2 (hmb)] 2 + CH 3 ONa CH 3 OTf Ru Ru NH 2 NH 2 S S S THF S CH 2 Cl 2 r.t NH 2 NH 2 HS r.t. NH 2 CH 3 Ru:SN = 1:2.2 KOt-C 4 H 9 2-propanol r.t. dimerization NH 2 S Ru:SN = 1:1 P-1 (#2) P2 1 / n (#14) R1 = 0.057 R1 = 0.064 wR2 = 0.148 wR2 = 0.148
Recommend
More recommend