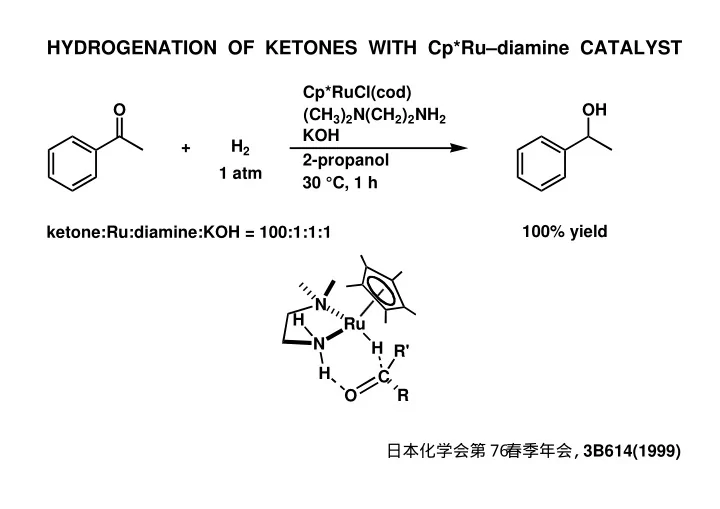

HYDROGENATION OF KETONES WITH Cp*Ru–diamine CATALYST Cp*RuCl(cod) O OH (CH 3 ) 2 N(CH 2 ) 2 NH 2 KOH H 2 + 2-propanol 1 atm 30 °C, 1 h 100% yield ketone:Ru:diamine:KOH = 100:1:1:1 N H Ru N H R' H C O R 日本化学会第7 6 春季年会 , 3B614(1999)
HYDROGENOLYSIS OF EPOXIDES WITH HOMOGENEOUS CATALYSTS K 3 [Co(CN) 5 ] O + H 2 OH C 6 H 5 C 6 H 5 C 6 H 6 –H 2 O, r.t. 1 atm 53% conv TOF = 0.64 h –1 S/C = 2.9, [ketone] = 0.34 M Kwiatek, Mador, Seyler, J. Am. Chem. Soc. , 1962, 84 , 304. [Rh(nbd)(P(C 2 H 5 ) 3 ) 2 ] + O C 6 H 5 H 2 + OH + C 6 H 5 C 6 H 5 diglyme–H 2 O O 1 atm 28 °C, 6 h n 90% conv 3 : 1 S/C = 100, [ketone] = 0.1 M Fujitsu, Mochida, J. Org. Chem., 1981, 46 , 2287.
LIGAND ACCELERATION IN HYDROGENOLYSIS OF EPOXIDES Cp*RuCl(cod) ligand OH O OH KOH + H 2 + 2-propanol 10 atm 30 °C, 2 h ketone:Ru:ligand:KOH = 100:1:1.5:1 branch/linear ligand: (C 6 H 5 ) 2 P NH 2 (C 6 H 5 ) 2 P NHCH 3 (C 6 H 5 ) 2 P N(CH 3 ) 2 63% conv 15% 5% >99/1 >99/1 89/11 (C 6 H 5 ) 2 P P(C 6 H 5 ) 2 C 6 H 5 CH 2 NH 2 + P(C 6 H 5 ) 3 (CH 3 ) 2 N NH 2 <1% <1% 0%
HYDROGENOLYSIS OF TERMINAL EPOXIDES Cp*RuCl(cod) (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH O KOH OH + H 2 + R 2-propanol R R 50 °C, 18 h branch/linear 10 atm substrate:Ru:ligand:KOH = 100:1:1.5:1 substrate: O O O C 6 H 5 C 6 H 5 100% conv 100% conv 90% conv 89/11 >99/1 >99/1 intact O O O C 6 H 5 99% conv 99% conv 98/2 >99/1
A POSSIBLE MECHANISM Ru Ar H P Ar H H 2 HN Cp*RuCl(cod) + Ru Ru Ar (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 Ar P H Ar P HN N H + Ar 2 KOH C 6 H 5 C 6 H 5 Ru Ar OH O H P Ar N H C 6 H 5 O H
HYDROGENOLYSIS OF ( S )-STYRENE OXIDE Cp*RuCl(cod) OH O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KOH + H 2 + 2-propanol 10 atm 50 °C, 18 h 100% conv 89 : 11 96% ee 0% ee substrate:Ru:ligand:KOH = 100:1:1.5:1
RACEMIZATION OF CHIRAL ALCOHOLS Cp*RuCl(cod) OH OH (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 KOH 2-propanol 50 °C, 30 min S/C = 100 99% ee 0% ee solvent effect 2-propanol > ethanol > methanol
HYDROGENOLYSIS OF ( S )-STYRENE OXIDE IN VARIOUS SOLVENTS Cp*RuCl(cod) (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH O KOH OH + H 2 + solvent 10 atm 50 °C 96% ee substrate:Ru:ligand:KOH = 100:1:1.5:1 conv, % ee, % solvent time, h branch/linear 2-propanol 1 79 89/11 0 ethanol 2 64 90/10 81 methanol 18 64 96/4 84
Cp*Ru(II) COMPLEXES BEARING PRIMARY AMINE LIGANDS Ar Ar P N H H Ru Ru N N H H R' H H CH 2 C O R O R Hydrogenation of ketones Hydrogenolysis of epoxides Racemization of secondary alcohols
Recommend
More recommend