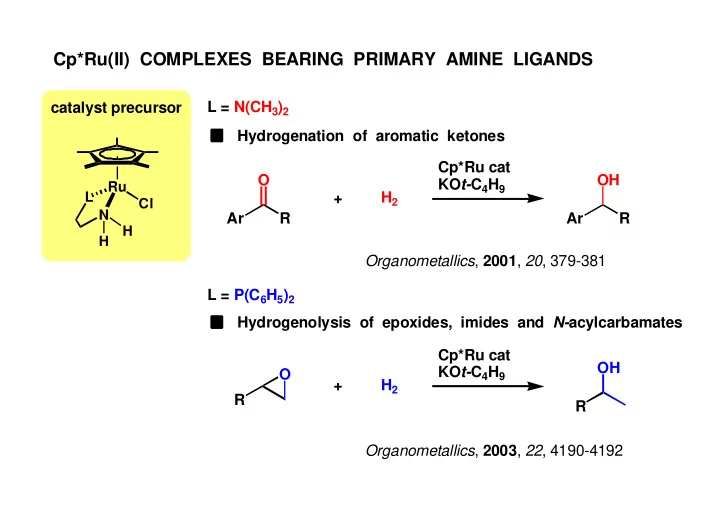

Cp*Ru(II) COMPLEXES BEARING PRIMARY AMINE LIGANDS L = N(CH 3 ) 2 catalyst precursor Hydrogenation of aromatic ketones Cp*Ru cat O OH KO t- C 4 H 9 Ru L H 2 + Cl N Ar R Ar R H H Organometallics , 2001 , 20 , 379-381 L = P(C 6 H 5 ) 2 Hydrogenolysis of epoxides, imides and N- acylcarbamates Cp*Ru cat OH KO t- C 4 H 9 O + H 2 R R Organometallics , 2003 , 22 , 4190-4192
SUMMARY Hydrogenolysis of epoxides with Ru catalysts cat OH KO t- C 4 H 9 O + H 2 2-propanol n- C 6 H 13 n- C 6 H 13 Ru Ph 2 P 10 atm 30 °C, 4 h H N >99% conv H Brønsted acidity of coordinated NH 2 group H Nucleophilicity of metal hydride Alcoholysis of epoxides with Rh, Ir catalysts (OTf) 2 O OH cat O OH + M 2-propanol C 6 H 5 C 6 H 5 Ph 2 P NCCH 3 30 °C, 4 h N major product H H >99% conv M = Rh, Ir Lewis acidity of metal center Brønsted acidity of coordinated NH 2 group
HYDROGENOLYSIS OF STYRENE OXIDE WITH [Cp*M(CH 3 CN)(Ph 2 PCH 2 CH 2 NH 2 )](OTf) 2 Rh, Ir cat O OH KO t- C 4 H 9 O + + H 2 OH O 2-propanol C 6 H 5 C 6 H 5 C 6 H 5 30 °C, 4 h α β epoxide:cat:KO t -C 4 H 9 = 100:1:1, [epoxide] = 0.5 M in 2-propanol α : β a conv, % a entry cat H 2 , atm catalyst precursor hydrogenated Ru b /KO t- C 4 H 9 1 10 >99 product c (OTf) 2 Rh/KO t- C 4 H 9 2 10 >99 95:5 Ph 2 P 3 Rh 0 >99 93:7 M 4 Ir/KO t- C 4 H 9 10 >99 91:9 N NCCH 3 H 2 5 Ir 0 >99 93:7 M = Rh, Ir 6 KO t- C 4 H 9 0 <1 a determined by 1 H NMR. b [Cp*Ru(CH 3 CN)(Ph 2 PCH 2 CH 2 NH 2 )](OTf). c C 6 H 5 CH(CH 3 )OH:C 6 H 5 CH 2 CH 2 OH = 78:22.
HYDROGENOLYSIS OF 1,2-EPOXYOCTANE WITH [Cp*Ru(CH 3 CN)(L — NH 2 )]OTf catalyst precursor cat OH OTf KO t- C 4 H 9 O + H 2 L 2-propanol n- C 6 H 13 n- C 6 H 13 10 atm 30 °C, 4 h Ru N NCCH 3 H 2 epoxide:cat:KO t- C 4 H 9 = 100:1:1, [epoxide] = 0.5 M in 2-propanol N(CH 3 ) 2 N PPh 2 PPh 2 PPh 2 PPh 2 L—NH 2 NH 2 NH 2 NH 2 NH 2 NH 2 NH 2 conv* <1 <1 71 21 >99 87 increase electron density on Ru center increase Brønsted acidity of coordinated NH 2 group *determined by 1 H NMR
ELECTRONIC PROPERTY OF Cp*Ru, Rh, Ir COMPLEXES BEARING P,N-LIGAND (OTf) 2 (OTf) 2 Ph 2 Ph 2 P P + M M CO CH 2 Cl 2 N N CO NCCH 3 1 atm 30 °C, 24 h H 2 H 2 M = Rh, Ir (OTf) 2 (OTf) 2 OTf Ph 2 Ph 2 Ph 2 P P P Rh Ir Ru N N N CO CO CO H 2 H 2 H 2 IR ( ν C ≡ O ) 2099 cm -1 2071 cm -1 1948 cm -1
SELECTED DATA FOR [Cp*Ru(CO)(L–NH 2 )]OTf OTf OTf Ph 2 Ph 2 P P + Ru Ru CO CH 2 Cl 2 N N CO NCCH 3 30 °C, 10 min 1 atm H 2 H 2 92% yield Ph 2 Ph 2 N P P Ru Ru Ru N N CO CO CO N H 2 H 2 H 2 IR ( ν C ≡ O ) 1951 cm -1 1947 cm -1 1938 cm -1 Ph 2 Me 2 Ph 2 P N P Ru Ru Ru N N N CO CO CO H 2 H 2 H 2 IR ( ν C ≡ O ) 1948 cm -1 1939 cm -1 1931 cm -1
PREPARATION OF [Cp*M(CH 3 CN)(Ph 2 PCH 2 CH 2 NH 2 )] n+ OTf OTf Ph 2 PPh 2 P CH 3 CN + Ru Ru CH 2 Cl 2 N CH 3 CN NCCH 3 NCCH 3 NH 2 30 °C, 1 h H 2 >99% yield (OTf) 2 Cl Ph 2 Ph 2 P P M 2 AgOTf M + CH 3 CN N N NCCH 3 Cl H 2 30 °C, 24 h H 2 >99% yield M = Rh, Ir
Cp*Ru(II) — AMINE CATALYSTS FOR THE REDUCTION OF POLAR BONDS H 2 Ru Ru L H L N NH H A B A B H L = P, N H H catalyst precursor hydride δ - nucleophilicity L H Ru δ + B N Ru A H L H δ - Cl δ + N proton H electrophilicity H
Recommend
More recommend