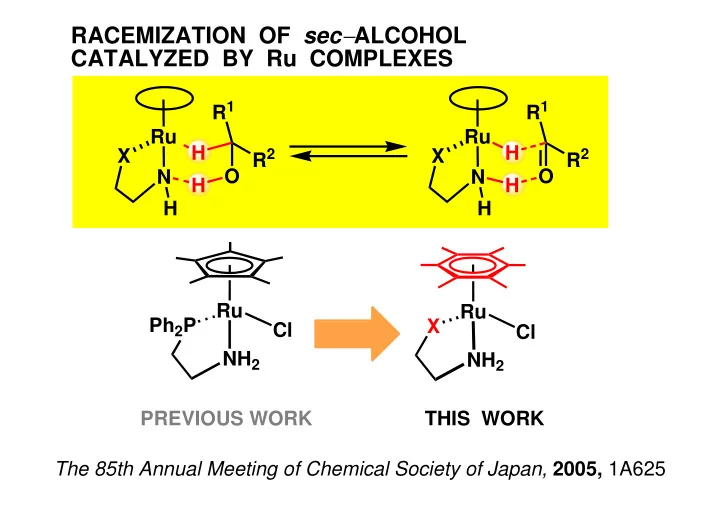

RACEMIZATION OF sec − ALCOHOL CATALYZED BY Ru COMPLEXES R 1 R 1 Ru Ru H H X X R 2 R 2 N O N O H H H H Ru Ru Ph 2 P X Cl Cl NH 2 NH 2 PREVIOUS WORK THIS WORK The 85th Annual Meeting of Chemical Society of Japan, 2005, 1A625
LIGAND EFFECT: CHELATING prim- AMINE OH OH [RuCl 2 (hmb)] 2 amine ligand Cl Cl KO t- C 4 H 9 Ru Ru Cl toluene, 30 °C Cl 24 h >99% ee S/C = 100 [RuCl 2 (hmb)] 2 amine ligand: SN chelate PhS NH 2 HS NMe 2 NH 2 HS 91 99 7 ee, % O O O S other HO NH 2 HO NH 2 TsN NH 2 HO NH 2 H ee, % 14 58 76 86
LIGAND EFFECT: ARENE R n [RuCl 2 (arene)] 2 OH OH HS(CH 2 ) 2 NH 2 Cl Cl KO t- C 4 H 9 Ru Ru R n Cl toluene, 30 °C Cl 24 h >99% ee S/C = 100 [RuCl 2 (arene)] 2 arene ligand 99 94 99 ee, % arene ligand ee, % 92 97 7
REACTION OF [RuCl 2 (hmb)] 2 WITH SN LIGAND NH 3 – Isolation of catalyst precursor – Cl 2 H 2 N Cl S 1/2 [RuCl 2 (hmb)] 2 + HS + 1/2 KO t- C 4 H 9 1/2 Ru NH 2 Ru 2-propanol S 30 °C 91% yield Bond lengths (Å) Ru(1) –Ru(2) 3.6790 Bond angles (°) Ru(1) –N(1) –C(14) 110.2 Ru(1) –S(1) –Ru(2) 100.6 Ru(1) –S(2) –Ru(2) 99.7 1 H NMR ( δ , CDCl 3 ) : 1.98 (s, 18H) 2.17 (s, 18H) P-1 (#2) R1 = 0.057 wR2 = 0.148
EFFECT OF STOICHIOMETRY AND REACTION CONDITIONS Cl S [RuCl 2 (hmb)] 2 N SN (excess) Ru Ru (excess) NH 2 H Ru Ru S S CH 3 ONa KOH H HS NH 2 THF NH 2 2-propanol 30 °C 30 °C 78% yield 68% yield P-1 (#2) P-1 (#2) R1 = 0.079 R1 = 0.120 wR2 = 0.318 wR2 = 0.188 Bond lengths (Å) Ru(1) –S(1) 2.383 Bond lengths (Å) Ru(1) –Ru(2) 2.7328 Ru(1) –S(2) 2.409 Bond angles (°) Ru(1) –N(1) –Ru(2) 82.5 Bond angles (°) Ru(1) –N(1) –C(14) 115.1 Ru(1) –S(1) –Ru(2) 70.51 Ru(1) –S(2) –C(15) 113.4 1 H NMR ( δ , CDCl 3 ): 2.27 (s, 36H) Ru(1) –S(1) –C(13) 99.7 –12.3 (s, 1H) 1 H NMR ( δ , CDCl 3 ): 2.01(s, 18H)
RACEMIZATION WITH THE Ru 2 (SN) 2 COMPLEX OH OH Ru cat KO t- C 4 H 9 toluene, 30 °C 6 h >99% ee S/C = 100 Ru cat KO t- C 4 H 9 / Ru ee, % 0 >99 NH 3 0.5 >99 Cl 2 H 2 N Cl 1 98 S Ru Ru 6 1.5 S 2 9 0 2.5 1/2 [RuCl 2 (hmb)] 2 + HSCH 2 CH 2 NH 2 2 36
RACEMIZATION WITH THE Ru 2 (SN) OR Ru(SN) 2 COMPLEXES OH OH Ru cat KO t- C 4 H 9 toluene, 30 °C 24h >99% ee S/C = 100 Ru cat KO t- C 4 H 9 / Ru ee, % 0 98 Ru NH 2 S S 2 97 NH 2 Ru(SN) 2 low reactivity Cl S N 0 99 H Ru Ru H 7 83 Ru 2 (SN) 2 7 1/2 [RuCl 2 (hmb)] 2 + HSCH 2 CH 2 NH 2
POSSIBLE MECHANISM NH 3 Cl 2 Cl 2 H 2 N Cl S NH 2 Cl Cl HS NH 2 Ru S Ru Ru Ru Ru Ru base base Cl S H 2 N Cl S base O OH R 1 R 2 R 1 R 2 S NH Ru Ru Ru Ru S H S HN NH S NH 2 O OH 16e 18e R 1 R 2 R 1 R 2 ESI-MS: m/z 340[M+H] + C 14 H 23 N 1 S 1 Ru 1
SUMMARY Racemization of sec- alcohols catalyzed by RuSN(hmb) complexes NH 3 OH OH Cl 2 H 2 N Cl Ru cat (0.5 mol%) S KO t- C 4 H 9 (1.5 mol%) Ru Ru toluene, 30 °C S 6 h >99% ee 6% ee Ru cat Isolation of catalyst precursors (hmb)Ru complexes with SN ligand Ru 2 (SN) Ru(SN) 2 Ru 2 (SN) 2
DYNAMIC KINETIC RESOLUTION (DKR) OF CHIRAL RACEMIC sec – ALCOHOLS O OH slow O R + acyl donor R 1 R 2 lipase R 1 R 2 Ru cat Dynamic Kinetic Resolution (DKR) O OH fast O R + acyl donor R 1 R 2 lipase R 1 R 2 Ph Ph O O Ph Ph Ph Ph Ph H NH Ph Ph Ph Ph Ph Ph Ph Ph H Ph X = Cl Ph Ru Ru Ru Ru Br OC Cl OC X OC CO I CO OC CO CO Shvo (1986) + KO t- Bu + KO t- Bu B äckvall (1999) Park (2002) B äckvall (2004) Casey (2001)
Recommend
More recommend