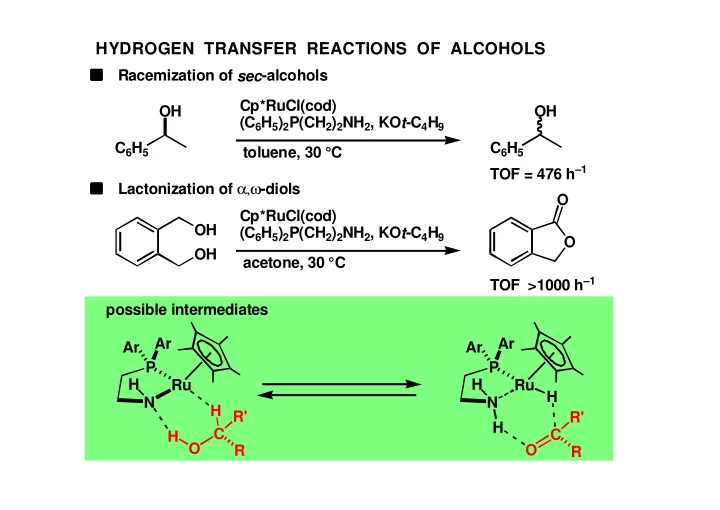

HYDROGEN TRANSFER REACTIONS OF ALCOHOLS Racemization of sec -alcohols Cp*RuCl(cod) OH OH (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 , KO t -C 4 H 9 C 6 H 5 C 6 H 5 toluene, 30 °C TOF = 476 h –1 Lactonization of α,ω -diols O Cp*RuCl(cod) OH (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 , KO t -C 4 H 9 O OH acetone, 30 °C TOF >1000 h –1 possible intermediates Ar Ar Ar Ar P P H H Ru Ru H N N H R' R' H C C H O R O R
THIS WORK H Ru Cl N R 1 PAr 2 R 2 R 3 Synthesis of Cp*Ru complexes bearing chiral P–N ligands Isolation of Cp*RuH(P–N) complex Enantioselective lactonization
SYNTHESIS OF OPTICALLY ACTIVE P–N LIGANDS H H 2 N P(C 6 H 5 ) 2 R = CH 3 83% yield O = CH(CH 3 ) 2 89 S + = CH 2 CH(CH 3 ) 2 85 R R 1 N O = C(CH 3 ) 3 66 = C 6 H 5 70 S = CH 2 C 6 H 5 70 R 2 HP(C 6 H 5 ) 2 NH P(C 6 H 5 ) 2 55% yield S 日本化学会第 81 春季年会, 3G414
SYNTHESIS OF OPTICALLY ACTIVE P–N LIGANDS O H 2 N PR 2 K 2 CO 3 aq CF 3 SO 3 H + HPR 2 HN O S toluene R 2 R 1 reflux, 24 h S R 1 R 2 H 2 N PR 2 R = p -CH 3 C 6 H 4 69% yield S 3,5-(CH 3 ) 2 C 6 H 3 66% C 6 H 5 H 2 C C 6 H 11 <1% H 2 N P(C 6 H 5 ) 2 R 2 = CH 3 77% yield S R 2 C 6 H 5 C 6 H 5 54% Conditions; oxazolidinone:HPAr 2 :CF 3 SO 3 H = 1:2:3
SYNTHESIS OF Cp*RuCl(P–N) COMPLEXES R 1 HN PAr 2 H Ru Cp*RuCl(isoprene) + Cl CH 2 Cl 2 R 1 N PAr 2 R 2 R 3 R 2 1:1 R 3 yield, % Ar R 1 , R 2 , R 3 31 P NMR C 6 H 5 H, H, H 99 60.9 C 6 H 5 57.1 H, CH 3 , H 92 C 6 H 5 H, i -Pr, H 88 55.8 56.6 C 6 H 5 H, i -Bu, H 82 55.2 H, t -Bu, H 85 C 6 H 5 56.3 88 C 6 H 5 H, Bn, H C 6 H 5 89 56.5 H, C 6 H 5 , H 59.6, 61.8 C 6 H 5 -(CH 2 ) 3 -, H 86 C 6 H 5 H, C 6 H 5 , C 6 H 5 70.1 82 54.0 p -CH 3 C 6 H 4 H, Bn, H 93 54.8 H, Bn, H 90 3,5-(CH 3 ) 2 C 6 H 3
SYNTHESIS OF Cp*RuCl(P–N) COMPLEX P–N Ru1 S Cl1 R Ru1 H 2 N PAr 2 H2 H1 H1 N1 P1 H2 Cl1 Bn N1 S P1 S Ar = p -CH 3 C 6 H 4 Ar = 3,5-(CH 3 ) 2 C 6 H 3 P 2 1 2 1 2 1 (#19), R1 0.078, wR2 0.202 P 1 (#1), R1 0.029, wR2 0.077 Selected bond lengths [Å] and angles [°] a Selected bond lengths [Å] and angles [°] Ru(1)—N(1) 2.1915(3) Ru(1)—N(1) 2.183(6) Ru(1)—P(1) 2.2885(10) Ru(1)—P(1) 2.302(2) Ru(1)—Cl(1) 2.495(2) Ru(1)—Cl(1) 2.4860(10) N(1)–Ru(1)–P(1) 79.7(2) N(1)–Ru(1)–P(1) 81.25(7) 79.58(9) N(1)–Ru(1)–Cl(1) 83.7(2) N(1)–Ru(1)–Cl(1) Cl(1)–Ru(1)–P(1) 91.49(5) Cl(1)–Ru(1)–P(1) 93.54(9) a average of two indipendent molecules
SYNTHESIS OF Cp*RuCl(P–N) COMPLEX H N P(C 6 H 5 ) 2 Ru H Cp*RuCl(isoprene) + Cl CH 2 Cl 2 N P(C 6 H 5 ) 2 r.t. 0.9 1.0 Ru1 S –90 °C N1 P1 Cl1 1.0 H 1.0 S P 2 1 /c (#14) 50 R 0.063 R W 0.062
SYNTHESIS OF Cp*RuCl(P–N) COMPLEX H 2 N P(C 6 H 5 ) 2 Ru Ru Cp*RuCl(isoprene) + + Cl Cl CH 2 Cl 2 H 2 N H 2 N P(C 6 H 5 ) 2 P(C 6 H 5 ) 2 S P2 Ru2 Cl2 S N1 N2 P1 Ru1 R Cl1 P 2 1 (#4) R1 0.028 wR2 0.083
EPIMERIZATION OF RUTHENIUM CENTRAL CHIRALITY 31 P NMR r.t. Ru Cl H 2 N P(C 6 H 5 ) 2 –50 °C 13.0 1.0 Ru Cl H 2 N P(C 6 H 5 ) 2 –90 °C 10.0 1.0 59 58 57 56
REACTION OF Cp*RuCl(P–N) COMPLEX KOH CO (1 atm) Ru Ru Ru Cl CO H 2 N HN HN P(C 6 H 5 ) 2 P(C 6 H 5 ) 2 P(C 6 H 5 ) 2 98% yield 13 C NMR CO 1 H NMR(CD 2 Cl 2 ) 205.6 ppm, d 10.0 ppm, s, C 5 ( C H 3 ) 5 1.64 ppm, d J P-H = 16.4 Hz, C O 32.0, d, J CP = 27.5 Hz, P C H 2 J P-H = 1.7 Hz, 15H, C 5 (C H 3 ) 5 47.2, d, J CP = 5.4 Hz, N C H 2 methylene bridge FT-IR 96.1, d, J CP = 1.9 Hz, C 5 (CH 3 ) 5 2.05-2.15 ppm, m, 1H 1933 cm -1 , ν CO C 6 H 5 2.60-2.71, m, 1H 128.7 ppm, d, J CP = 10.3 Hz 2.87-3.01, m, 1H 129.2, d, J CP = 10.3 Hz 3.40-3.51, m, 1H 130.2, d, J CP = 2.7 Hz 6.63, br, 1H, N H 131.5, d, J CP = 2.7 Hz 7.22-7.62, m, 10H, aromatics 132.8, d, J CP = 10.7 Hz 31 P NMR 133.5, d, J CP = 49.6 Hz 64.5 ppm, s 134.4, d, J CP = 12.2 Hz 135.2, d, J CP = 42.7 Hz
REACTION OF Cp*RuH(P–N) COMPLEX WITH ACETONE KOH Ru Ru Cl H 2-propanol H 2 N H 2 N P(C 6 H 5 ) 2 P(C 6 H 5 ) 2 96% yield 1 H NMR(THF-d 8 ) -10.6 ppm, d, J PH = 40.3 Hz, 1H, Ru- H 1.9, d, J PH = 0.9 Hz, 15H, C 5 (C H 3 ) 5 3.0, m, 2H, methylene bridge 4.1, m, 2H, methylene bridge 7.4-8.2, m, 10H, aromatics 31 P NMR 79.4 ppm, s
EARLY EXAMPLES FOR Ru–CATALYZED LACTONIZATION O Ru 2 Cl 4 ((-)diop) 3 OH PhCH=CHCOCH 3 , 2 equiv OH O n toluene, reflux n S/C = 25 up to 12% ee Ishii, Osakada, Ikariya, Saburi, Yoshikawa, Chem. Lett. , 1982 , 1179. O [RuCl(( S )-binap)(benzene)]Cl OH O PhCH=CHCOCH 3 (2 equiv) OH toluene, SDS, 60 °C, 44 h 89% yield 11% ee S/C = 100 Nozaki, Yoshida, Takaya, J. Organomet. Chem. , 1994 , 473 , 253. Ru(salen) OH PDC, MS4A h υ O O OH n air, CHCl 3 CH 2 Cl 2 n n r.t., 2-3 d OH O S/C = 50 up to 67% ee Shimizu, Nakata, Katsuki, Chem. Lett. , 2002 , 1080.
ENANTIOSELECTIVE LACTONIZATION FROM meso -DIOLs Cp*RuCl(P–N) S OH KO t -Bu O OH acetone R 30 °C, 1 h O diol:Ru:chiral ligand:KO t -Bu = 100:1:1:1 R 1 , R 2 , R 3 ee, % Ar yield, % Cp*RuCl(P–N) Ph H, Me, H >99 43 Ph H, i -Pr, H >99 44 >99 41 Ph H, i -Bu, H H Ru 39 Ph H, t -Bu, H >99 Cl R 1 N Ph >99 10 -(CH 2 ) 3 -, H PAr 2 Ph >99 41 H, Bn, H R 2 R 3 p -CH 3 C 6 H 4 H, Bn, H >99 45 >99 3,5-(CH 3 ) 2 C 6 H 3 H, Bn, H 45 Ph >99 46 H, Ph, H
KINETIC RESOLUTION OF rac -1,4-BUTANDIOL Ar Ar Ar Ar Cp*RuCl(P–N) KO t -Bu OH OH OH O + + OH OH acetone OH toluene O Ar Ar Ar Ar 30 °C, 8 h 50:50 26% conv. 33% ee 92% ee (2 R , 3 S ) (2 S , 3 R ) k f / k s = 33.0 Ar = C 6 H 5 diol:acetone:Ru:chiral ligand:KO t -Bu = 100:100:1:1:1 Cp*RuCl(P–N) Ru Cl H 2 N P(C 6 H 5 ) 2 C 6 H 5
SUMMARY Synthesis of Cp*Ru complexes bearing chiral P–N ligands R 1 HN PAr 2 Ru H Cp*RuCl(isoprene) + Cl CH 2 Cl 2 R 1 N P(C 6 H 5 ) 2 R 2 R 3 1:1 R 2 R 3 Isolation of Cp*RuH(P–N) complex Enantioselective lactonization R 1 R 1 R 1 R 1 Cp*RuCl(PN) R R KO t -C 4 H 9 OH O OH R R O up to 92% ee
Recommend
More recommend