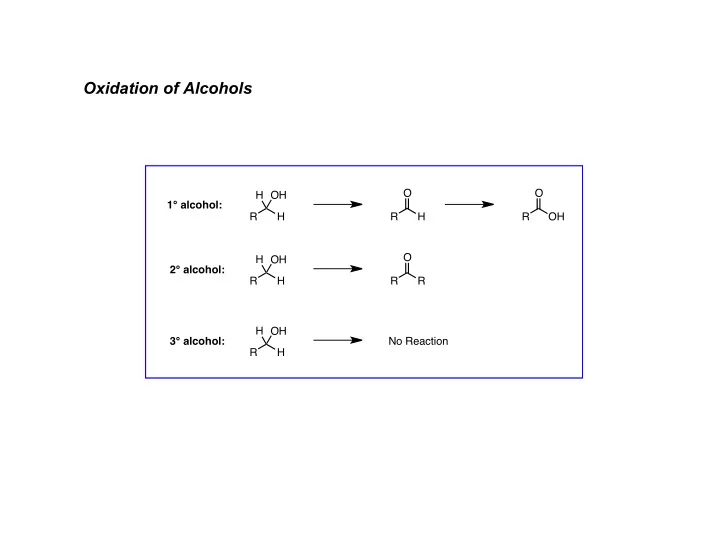

Oxidation of Alcohols O O H OH 1° alcohol: R H R H R OH O H OH 2° alcohol: R H R R H OH 3° alcohol: No Reaction R H
A. Chromium Based Reagents General Mechanism: � � � :B O H H H H slow O + O CrL n R OH R O Cr L n-1 R H O H OH H 3 O R R OH OH - 1° alcohols: under anhydrous conditions (Collins, PCC, PDC) will stop at aldehyde - in presence of aqueous acid (Jones), see further (rapid) oxidation to carboxylic acid - oxidation of 2° alcohols give ketones - these processes generate chromium waste (toxic)
A. Chromium Based Reagents 1. CrO 3 /H 2 SO 4 (aq) : Jones Oxidation � � � • preparation O O O H 2 O 2 HO Cr O Cr OH HO Cr OH CrO 3 + H 2 O + H 2 SO 4 O O O (dilute) (concentrated) - reagent is shelf stable • reactivity O CrO 3 , H 2 SO 4 acetone O OH O OH Yamamoto Tetrahedron 85% 1990 , 46 , 4595. - 1° alcohol CO 2 H - rapid reaction - strongly acidic; not useful for acid sensitive substrates - reaction can effectively be run as a titration
A. Chromium Based Reagents • mechanism O R H R H slow H 2 CrO 4 O + H 2 CrO 3 HOCrO R' OH R' O Cr OH acetone R R' Cr VI (red) O Cr III (green) R 2 CH-OH + Cr (VI) R 2 C=O + Cr (IV) + 2 H + R 2 CH-OH + Cr (IV) R 2 C=O + Cr (II) + 2 H + Cr (II) + Cr (VI) Cr (III) + Cr (V) R 2 CH-OH + Cr (V) R 2 C=O + Cr (III) + 2 H + - stoichiometry: 3 R 2 CHOH + 2 CrO 3 + 6 H + 3 R 2 C=O + 2 Cr 3+ + 6 H 2 O
A. Chromium Based Reagents 2. CrO 3 •pyridine : Collins reagent � � � • preparation N O hygroscopic H 2 O (Cr 2 O 7 ) 2- (pyrH + ) 2 O Cr CrO 3 + 2 pyridine red crystalline O solid N (yellow) - important: add CrO 3 to pyridine (reverse results in strong exotherm!) - Sarett: in situ generation in pyridine - Collins: isolated solid; reaction in CH 2 Cl 2 - Radcliff: in situ generation in CH 2 Cl 2 • reactivity OH O CrO 3 , pyr CH 2 Cl 2 H H 95% Ratcliffe JOC 1970 , 35 , 4000. - 1° alcohol CHO - neutral to slighlty basic; good for acid sensititve substrates - requires large excess of reagent; anhydrous conditions
A. Chromium Based Reagents 3. Pyridinium Chlorochromate (PCC) : Corey-Suggs Oxidation � � • preparation N O orange solid CrO 3 + HCl + pyridine H Cr O Cl O - stable; commercially available - chloride facilitates formation of chromate ester • reactivity O O PCC 4Å MS, CH 2 Cl 2 O OH O O Nicolaou J. Am. Chem. Soc. 1988 , 110 , 4672 94% - 1° alcohol CHO - can use in near stoichiometric amounts (ca. 1.5 equiv) - mild conditions; slightly acidic can buffer with NaOAc - add powd MS or Celite to facilitate product isolation - addition of MS can accelerate rxn rate - can promote allylic rearrangements
A. Chromium Based Reagents 4. Pyridinium Dichromate (PDC) : Corey-Schmidt Oxidation � � • preparation orange solid CrO 3 + pyridine + H 2 O N 2 Cr 2 O 72- H - stable; commercially available • reactivity PDC PDC CO 2 H O CH 2 Cl 2 DMF OH Corey Tetrahedron Lett. 1970 , 20 , 399. - product of reaction depends on solvent used CH 2 Cl 2 : 1° alcohol CHO DMF: 1° alcohol CO 2 H (allylic alcohols give CHO) - oxidizes more slowly than other Cr-based reagents - mild conditions; less acidic than PCC
B. Manganese Based Reagents 1. Manganese Dioxide (MnO 2 ) � • reagent - dark brown or black solid - structure/activity depends on preparation - non-stoichiometric material containes Mn(II) and Mn(III) oxides and hydrated species • reactivity OH O MnO 2 MeO MeO OH OH acetone MeO MeO - selective oxidation of allylic and benzylic alcohols; significant rate difference! - 1° alcohol CHO - slow reaction, requires large excess of reagent - H bonding solvents show strong deactivating effect; non-polar solvents best - mild; no isomerization of double bonds upon oxidiation of allylic alcohols
B. Manganese Based Reagents 2. Manganese Dioxide, ROH, NaCN : Corey-Gilman-Ganem Oxidation � � • reagent - modified MnO 2 oxidation • reactivity MnO 2 , NaCN O O MeOH, AcOH CO 2 Me OH - direct oxidation of 1° allylic/benzylic alcohols to esters - more commonly used for the conversion of conjugated aldehydes to esters
B. Manganese Based Reagents 3. Potassium Permanganate (KMnO 4 ) • reactivity O CN O CN KMnO 4 , NaH 2 PO 4 tBuOH, H 2 O CHO CO 2 H N N Joullié J. Am. Chem. Soc. 1992 , 114 , 10181. 94% Boc Boc - 1° alcohol CO 2 H; also useful for the oxidation of aldehydes - powerful oxidant; over oxidation/side reactions may be a problem also oxidizes alkenes, 1,2-diols, etc. - insoluble in organic solvents - may be successful when other oxidants fail (Jones, AgO, NaOCl). - R 4 NMnO 4 shows similar reactivity and is soluble in organics
C. Ruthenium Based Reagents 1. Ruthenium Tetraoxide (RuO 4 ) • reagent - toxic - catalytic procedures use 1-5% Ru metal with a stoichiometric oxidant • reactivity O O RuCl 3 -NaIO 4 MeCN, CCl 4 , H 2 O H H 60% OBz OBz Overman J. Am. Chem. Soc. O HO 1997 , 119 , 12031. HO - 1° alcohol CO 2 H - powerful, non-selective oxidant; will also attack multiple bonds,1,2-diols, ethers, aromatic rings, etc .
C. Ruthenium Based Reagents 2. Tetra-n-propylammonium Perruthenate (Pr 4 N + RuO 4 - ): TPAP • reagent - developed by Steve Ley (Imperial College Cambridge) - catalytic; used in conjunction with a stoichiometric oxidant (NMO) - perruthate salts with a large counterion are mild and selective oxidants • reactivity CBz CBz N N TPAP, NMO 4Å MS, CH 2 Cl 2 O HO Jacobsen J. Am. Chem. Soc. 2004 , 126 , 706. - 1° alcohol CHO - mild oxidant; no over oxidation, does not react with multiple bonds - use of MS required to remove water and achieve high catalyst turnover - modified conditions allow for oxidation of 1° alcohol to carboxylic acid (Stark Org. Lett. 2011 , 13 , 4164)
C. Ruthenium Based Reagents 2. Tetra-n-propylammonium Perruthenate (Pr 4 N + RuO 4 - ): TPAP • mechanism http://www.synarchive.com/named-reactions/Ley-Griffith_Oxidation
D. DMSO Based Reagents General Mechanism: � � � R OH B H E CH 2 S O E S O R O S Me H Me CH 2 + R S O R O S Me Me - mild class of reagents - don’t have environmental issues associated with use of Cr based reagents - no over oxidation oxidation of 1° alcohols give aldehydes - oxidation of 2° alcohols give ketones
D. DMSO Based Reagents 1. DMSO, (COCl) 2 ; Et 3 N: Swern Oxidation � � • activation: Cl O O Me S Cl + Cl Cl S S O O Cl Me O O + CO 2 + CO + Cl - - also TFAA, Ac 2 O, SOCl 2 , Cl 2 , P 2 O 5 • reactivity O O DMSO, (COCl) 2 CH 2 Cl 2 ; then Et 3 N Funk J. Org. Chem. OH CHO 1987 , 52 , 3173. - 1° alcohol CHO - most common of DMSO based reagents - very mild run at low temp (-78 to -60°C) - low sensitivity to steric factors - preparation of β -alkoxy carbonyl derivatives may be problematic use Et 2 NiPr
D. DMSO Based Reagents 2. DMSO, DCC, TFA, pyridine: Moffatt Oxidation � � • activation: DMSO + DCC S O + S O N C N N C N • reactivity OBz OBz OH O DMSO, EDC TFA, pyr OBPS OBPS Hannessian Can. J. Chem. MeO O MeO O 1981 , 59 , 870. 94% - 1° alcohol CHO - first reported DMSO based oxidant; less commonly used - separation of by-pyroduct (dicyclohexylurea) can be difficult use EDC N C N HCl•Me 2 N - may result in formation of MTM ethers (side reaction)
D. DMSO Based Reagents 3. SO 3 •pyridine, DMS; Et 3 N: Parikh-Doehring � � • activation O O + S O S O S O O S O O • reactivity H H H H O O SO 3 •pyr, DMSO O HO CH 2 Cl 2 ; Et 3 N H H Evans ACIEE 1999 , O O H H 38 , 3175 Br Br - 1° alcohol CHO - easy workup; well suited to large scale reactions
E. Silver Based Oxidants 1. Ag 2 CO 3 /celite: Fetizon’s reagent � � • reactivity MeO MeO Ag 2 CO 3 O O toluene, 110° NMe 84% NMe Rappoport - codeine HO O - 1° alcohol CHO - original oxidant modified by Fetizon adsorb on celite to increase surface area - neutral conditions; very sensitive to steric factors - $$$, must use large excess small scale reactions - reaction does not proceed through cationic intermediate (no rearrangements, etc.) - controlled overoxidation possible with some substrates (selective lactol oxidation) MeO MOMO OBn HO MeO MOMO OBn Ag 2 CO 3 /celite benzene, 80°C O OH O Kallmerten Tetrahedron Lett. 1990 , 31 , 4305.
E. Silver Based Oxidants 2. Silver (I) Oxide (Ag 2 O) � � • reactivity CHO CO 2 H Ag 2 O EtOH (aq) HO HO Kitching JCSP1 1995 , 1309. 80% - mild method for the conversion of CHO CO 2 H (in presence of free OH) - unsaturated aldehydes are problematic (isomerization) - weak oxidant
F. Other Oxidants 1. Dess-Martin Periodinane � � • preparation O O CO 2 H Ac 2 O KBrO 3 O O pTsOH, 100°C H 2 SO 4 I I I OAc O AcO HO OAc (IBX) shock white sensitive solid - can determine quality of reagent by solublity in CH 2 Cl 2 • reactivity O O Dess-Martin O O CH 2 Cl 2 Danishefsky J. Am. Chem. Soc. MeO MeO CHO 1991 , 113 , 3850. OH - 1° alcohol CHO - mild reagent; nearly neutral conditions gives off AcOH, but can buffer - will not oxidize N or S
F. Other Oxidants • mechanism O O O O O O AcO AcO O - AcOH I I I AcO AcO AcO + + 2 AcOH AcO O H R OH H R H H R H - addition of 1 equiv water accelerates reaction (Schreiber)
Recommend
More recommend