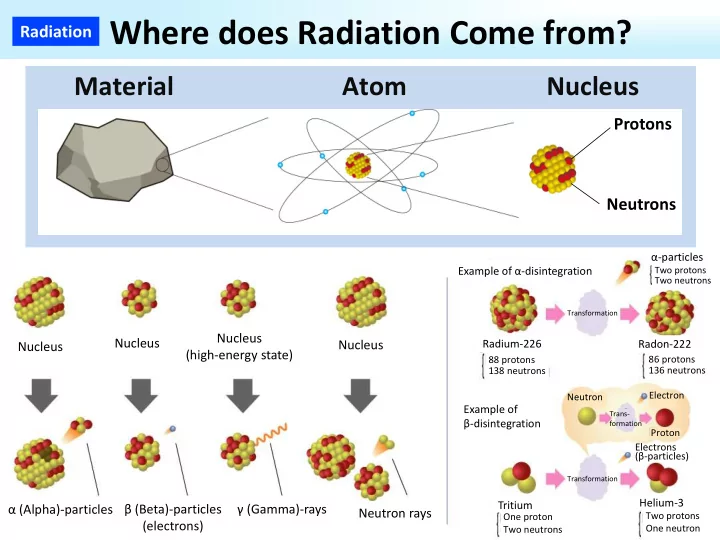

Radiation Where does Radiation Come from? Material Atom Nucleus Protons Neutrons α‐particles Example of α‐disintegration Two protons Two neutrons Transformation Nucleus Nucleus Nucleus Radium‐226 Radon‐222 Nucleus (high‐energy state) 86 protons 88 protons 136 neutrons 138 neutrons Electron Neutron Example of Trans‐ β‐disintegration formation Proton Electrons (β‐particles) Transformation Helium‐3 Tritium α (Alpha)‐particles β (Beta)‐particles γ (Gamma)‐rays Neutron rays Two protons One proton (electrons) One neutron Two neutrons
Radiation Types of Radiation Ionizing Particle Charged‐particle α‐particles (helium nuclei ejected from a nucleus) radiation beams beams (directly β‐particles (electrons ejected from a nucleus) ionizing radiation) Proton beams, deuteron beams, triton beams, heavy‐ion beams Charged meson beams Fission fragments, etc. Uncharged particle Uncharged meson beams beams (indirectly Neutrino ionizing radiation) Neutron beams, etc. (produced in nuclear reactors, accelerators, etc.) Electromagnetic waves X‐rays (generated outside a nucleus) (indirectly ionizing radiation) γ‐rays (emitted from a nucleus) Electric waves, microwaves, infrared rays, visible rays, ultraviolet rays, etc. Nonionizing radiation While radiation includes ionizing radiation and nonionizing radiation, radiation usually means ionizing radiation. Partially revised "Ionizing Radiation" in the Encyclopedia for Public Acceptance of Atomic Energy Accessible on the Internet, ATOMICA
Radiation Types of Ionizing Radiation Ionizing radiation Radiation that causes ionization Radiation that causes ionization α‐particles (helium nuclei ejected from a Particle beams nucleus) β‐particles (electrons ejected from a Protons nucleus) Neutrons Neutron beams(produced in nuclear reactors, accelerators, etc.) Electrons Proton beams(produced in accelerators, Electromagnetic waves etc.) Electrons X‐rays (generated outside a nucleus) (β‐particles) * X‐rays generated when electrons within an atom are caused to travel between orbits by incident electrons are called characteristic X‐rays. γ‐rays (emitted from a nucleus)
Radiation X‐rays for Medical Use and Generators Structural drawing of an X‐ray generator Braking X‐rays Electrons Voltage V (several to hundreds of thousands V) (β‐particles) Thermal electrons Braking X‐rays Anode (Anticathode) Cathode Characteristic X‐rays Braking X‐rays Characteristic X‐rays Electrons Characteristic X‐rays Electron orbits
Radiation Types of Electromagnetic Waves Visible light Energy 1 10 10 10 8 10 6 10 4 10 2 10 -2 10 -4 10 -6 10 -8 10 -10 10 -12 (eV) Ultraviolet rays Electric waves X‐rays, γ‐rays (Generally, γ‐rays come from Ultrashort waves Medium waves Short waves Infrared within a nucleus, and X‐rays Microwaves Long waves rays come from outside a nucleus.) 1 10 -16 10 -14 10 -12 10 -10 10 -8 10 -6 10 -4 10 -2 10 2 10 4 10 6 (m) Wavelength 1 pm 1 nm 1 μm 1 mm 1 m 1 km • Light has particle properties in Direction of the electric field addition to wave properties. • Electromagnetic waves are called "photons" when they are considered as particles. The values indicated above show photons' energy Direction of (eV) and those indicated below show their the magnetic wavelengths (m) as wave motions. Direction of field propagation of pm: picometers μm: micrometers nm: nanometers eV: electron volts electromagnetic waves
Radiation Ionization of Radiation ‐ Property of Ionizing Radiation Ionization Electrons Radiation Separation into positive ions and negative electrons γ‐rays α‐particles Atoms turned into positive ions Ejected electrons
Radiation Types of Radiation and Biological Effects • α‐particles ‐ Two protons plus two neutrons + + ‐ Helium (He) nuclei High ionization density ‐ Charged particles (2+) • β‐particles - ‐ Electrons (or positrons) + Low ionization density ‐ Charged particles (‐ or +) • γ‐rays and X‐rays Low ionization density/high ‐ Electromagnetic waves (photons) penetrating power • Neutron beams ‐ Neutrons High ionization density ‐ Uncharged particles When the ionization number is the same, the higher the ionization density is, the larger the biological effects are.
Radiation Penetrating Power of Radiation Radiation can be blocked by various substances. Weaken γ‐rays and Block α‐particles Block β‐particles X‐rays α‐particles β‐particles γ‐rays and X‐rays Thin sheet of Thick sheet of Paper metal such as lead or iron aluminum Weaken neutron beams Neutron beams Substance containing hydrogen Such as water or concrete
Radiation Penetrating Power of Radiation within the Body Upon collision with Distance traveling the body in the air + α‐particles Several to several tens of 1 to 10 cm micro meters Particles (Helium nucleus) (One‐trillionth of a centimeter) Several meters Several β‐particles (depending on the amount millimeters of energy) Particles (electrons) Several tens of γ‐rays meters X‐rays Several centimeters ‐ (depending on the (depending on the amount of energy) amount of energy)
Radiation Penetrating Power and Range of Effects on the Human Body When radioactive materials are When radioactive materials are located outside the body located within the body Within the body Outside Within the body Outside the body the body Affected part α‐particles α‐particles Organs, β‐particles etc. β‐particles γ‐rays γ‐rays Radioactive materials Peripheral tissues in the tissues
Recommend
More recommend