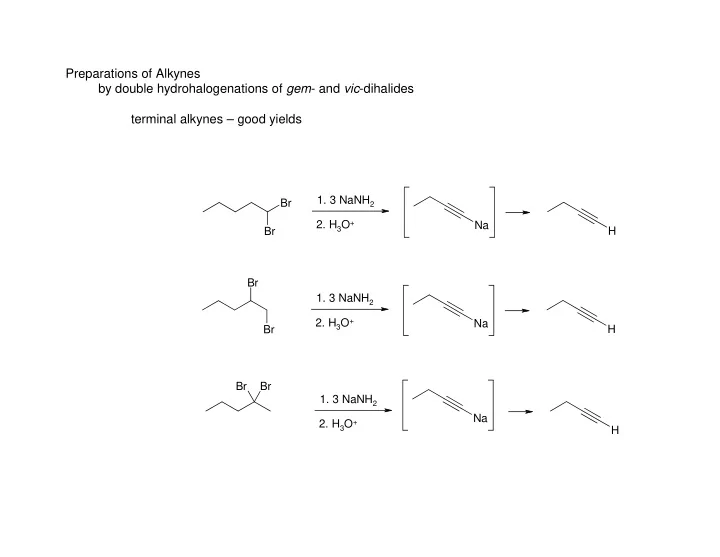

Preparations of Alkynes by double hydrohalogenations of gem - and vic -dihalides terminal alkynes – good yields 1. 3 NaNH 2 Br 2. H 3 O + Na Br H Br 1. 3 NaNH 2 2. H 3 O + Na Br H Br Br 1. 3 NaNH 2 Na 2. H 3 O + H
Preparations of Alkynes by double hydrohalogenations of gem - and vic -dihalides internal alkynes – usually lower yields than terminal alkynes, due to allene by-products and alkyne isomerization; alternative bases mitigate this Br 1. 3 NaNH 2 H + -NH 2 2. H 3 O + H Br H .. H H + .. - N C H - H H H H C H + -NH 2 H . . H .. C + - N - H H H H + NH 3 .. - + -NH 2 H
Preparations of Alkynes internal alkynes because of the above problem, internal alkynes usually are prepared not by elimination but by substitution : NaNH 2 Br .. - H
Naturally occurring alkynes (+)-Laurencin isolated from red algae Laurencia glandulifera Burton, J.W.; Clark, J.S.; Derrer, S.; Stork, TC.; Bendall, J.G.; Holmes, AB.; J. Am. Chem. Soc. 1997 , 119 , 7483.
Thiarubrine A � antiviral � antifungal ( Candida albicans ) � antibacterial Masato Koreeda, M.; Yang, W. J. Am. Chem. Soc. 1994 , 116 , 10793.
Enediynes Share a common ( Z )-1,5-diyn-3-ene moiety � Target minor groove or AT-rich DNA & intercalate � “Triggered” by an extended conjugate addition � Result of pulling trigger is a diradical � Diradical leads to SS and DS DNA cuts � NCZ diradical Nicolaou, K.C.; Dai, W.-M. Angew. Chem. Int. Ed. Engl. 1991 , 30 , 1387-1530.
Synthesize 2-heptyne from acetylene (ethyne) and any haloalkanes you need
Retrosynthesis: + .. - C7 Na + - Br C4 H C3
Now do the same thing for 1-propyne:
So the overall forward synthesis is a 2C + 1C + 4C coupling strategy : NaNH 2 CH 3 Br - Na + H H H C THF THF NaNH 2 Br - Na + C CH 3 H CH 3 THF THF CH 3
Now, provide a multistep synthesis of meso -5,6-decanediol from # 4C sources of carbon and any other reagents you require. CH 2 CH 2 CH 2 CH 3 H O H H O H CH 2 CH 2 CH 2 CH 3
CH 2 CH 2 CH 2 CH 3 Is a C10 vicinal diol. ˆ synthesis will involve chain elongation of an alkyne. H O H Diols are made from alkenes. Which alkene? H O H CH 2 CH 2 CH 2 CH 3 H O H H O H ( Z )-5-decene. How is ( Z )-5-decene made? From an alkyne: 5-decyne
Finally, how is this alkyne created from pieces of 4 carbons or fewer? Br + Br + So this is a 2C + 4C + 4C = 10C assembly strategy.
The forward synthesis of meso -5,6-decanediol is: . . .How would a synthesis of ( SR, SR )-5,6-butanediol differ from the one just proposed?
Synthesize heptanal from acetylene, # 5C compounds, and any other reagents required: O ? H H H acetylene heptanal One way: a 2C + 5C = 7C retrosynthetic strategy H O O is the keto form of H the enol: H H H H + Br H
Forward synthesis: NaNH 2 Br H Na H H H O 1. BH 3 @ THF H 2. H 2 O 2 , OH -
A second strategy: a [(2C + 4C) + 2C] - 1C = 7C approach H H O H H H H H H H Br + H H H Br + H
Forward synthesis: H 2 NaNH 2 Br H H H Na Lindlar Pd H NaNH 2 H H H H HBr H Na H Br ROOR H H O O H 2 1. O 3 H + H H H Lindlar Pd 2. Zn, H 3 O + H
Using the general strategy of our second synthesis, can you think of a third way that makes heptanal without generating any other aldehydes as side-products?
Recommend
More recommend