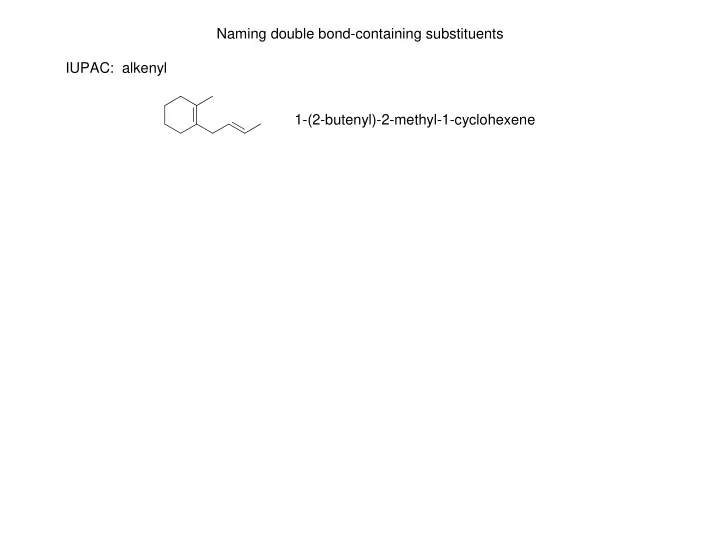

Naming double bond-containing substituents IUPAC: alkenyl 1-(2-butenyl)-2-methyl-1-cyclohexene
Naming double bond-containing substituents IUPAC: alkenyl 1-(2-butenyl)-2-methyl-1-cyclohexene Some common double bond-containing substituents: vinyl (ethenyl) 1-methyl-4-vinyl-1-cyclohexene
Naming double bond-containing substituents IUPAC: alkenyl 1-(2-butenyl)-2-methyl-1-cyclohexene Some common double bond-containing substituents: vinyl (ethenyl) 1-methyl-4-vinyl-1-cyclohexene allyl (2-propenyl) 1-allyl-4-vinyl-1-cyclohexene
Naming double bond-containing substituents IUPAC: alkenyl 1-(2-butenyl)-2-methyl-1-cyclohexene Some common double bond-containing substituents: vinyl (ethenyl) 1-methyl-4-vinyl-1-cyclohexene allyl (2-propenyl) 1-allyl-4-vinyl-1-cyclohexene phenyl 4,4-dimethyl-1-phenyl-1-cycloheptene
Naming double bond-containing substituents IUPAC: alkenyl 1-(2-butenyl)-2-methyl-1-cyclohexene Some common double bond-containing substituents: vinyl (ethenyl) 1-methyl-4-vinyl-1-cyclohexene allyl (2-propenyl) 1-allyl-4-vinyl-1-cyclohexene phenyl 4,4-dimethyl-1-phenyl-1-cycloheptene benzyl (phenylmethyl) ( R )-1-benzyl-4-methyl-4-phenyl-1-cycloheptene
When the parent chain contains multiple C=C, use a multiplicative prefix before “ene”: Cl ( S )-6-chloro-4-methyl-2,4-heptadiene
Alenes are classified according to their degree of substitution of the C=C functional group H monosubstituted H H H disubstituted H H terminally disubstituted H trisubstituted H tetrasubstituted
How strong is a C=C ? Is it twice the strength of a C–C? � � � H1s H1s � Csp 2 Csp 2 H1s H1s � � � H DBE , kcal/mol . . H 3 C CH 3 CH 3 CH 3 + 1 σ 83 1 σ + 1 π 146 H 2 C CH 2 :CH 2 :CH 2 + � � H BDE (1 σ + 1 π ) - � H BDE (1 σ ) = � H BDE (1 π ) 146 - 83 = 63
cis -alkenes and trans -alkenes Consider all the isomers of the alkene C 4 H 8 : are these conformational isomers? • No! They are isomers that differ by the relative positions of the substituent methyl groups in space. They cannot be converted into each other without breaking a bond . • • Therefore, they are diastereoisomers. • Why does rotation about the C=C break a bond?
H H H H C C C CH 3 CH 3 CH 3 H 3 C H C C C H CH 3 H 3 C π bond no π bond π bond reforms These diastereoisomers are named just like disubstituted cycloalkanes: Identical groups on the same side of the C=C (that is, with a 0 � dihedral angle) • are cis -alkenes Identical groups on opposite sides of the C=C (that is, with a 180 � dihedral • angle) are trans -alkenes
trans -2-butene cis -2-butene CH 3 H O Cl OH OH Br H trans -cinnamic acid ?
The E , Z system of naming diastereomeric alkenes: • Use the Cahn-Ingold-Prelog “Priority Rules” 1. For each C of the C=C, rank the priorities of the 2 atoms attached, according to atomic number. 2. If the 2 atoms attached are identical, rank the atoms attached to those 2 atoms. Priority of the entire substituent is determined by the first point of difference . 3. If identical priority numbers are on the same side of the C=C, the alkene is a ( Z )-alkene. 4. If identical priority numbers are on opposite sides of the C=C the alkene is a ( E )-alkene.
For comprison purposes, identical atoms must be compared in the same hybridization state: • The “Phantom atom” Rules 1. For naming purposes, a π bonded atom is considered to replicated by a phantom σ bond to a phantom atom. 2. Rule 1 is applied to both atoms of the π bond. 3. Rules 1 and 2 apply to each π bond of multiply bonded atoms. 4. All other things being equal, phantom atoms rank below real atoms of the same atomic number. X C Y Y C X C X Z Z
An example: Cl What is the IUPAC name of ? Cl 1. The longest chain containing both C=C bonds is 5: pentadiene 2. Number the chain to give the C=C bonds the lowest locants: 1,3-pentadiene 3. Add substituents: 1,5-dichloro-3-ethyl-4-methyl-1,3-pentadiene 1 4. Add stereodesignator for the C 1 =C 2 bond: C C Cl (1 E )-1,5-dichloro-3-ethyl-4-methyl-1,3-pentadiene C H 2 H C H 5. Add stereodesignator for the C 3 =C 4 bond: C 3 C C C 4 H H H H (1 E ,3 E )-1,5-dichloro-3-ethyl-4-methyl-1,3-pentadiene 2 Cl 1
Another example using the phantom atom rule: H H H C O C Is this an E or a Z alkene? C H H O
Another example using the phantom atom rule: 2 1 1 Is this an E or a Z alkene? 2 It’s an E alkene.
Thermodynamic stabilities of isomeric alkenes: Consider C 4 H 8 + 6 O 2 � 4 CO 2 + 4 H 2 O � H° rxn = � H° rxn kcal/mol CO 2 + H 2 O
The substitution - elimination continuum what factors influence the balance between substitution and elimination for a given class of haloakanes?
Primary haloalkanes: • S N 2 for all Z- • Increased steric hindrance in ‡ increases the proportion of E2 � -branching in haloalkane P strong, bulky Lewis bases P
Secondary haloalkanes: • S N 2 for all Z- less basic than HO- • E2 for all Z- more basic than HO- • Increased steric hindrance in ‡ increases the proportion of E2 � -branching in haloalkane P strong, bulky Lewis bases P • S N 1 or E1 when Z: is neutral and the solvent CA of Nu: pK a H-CN 9.2 H-OH 15.7 H-CH 2 CH 3 50
Tertiary haloalkanes: • E2 for all Z- • S N 1 or E1 for most Z: and the anions of strong CA Generally, regardless of the identity of Z: or Z- : • S N is favored at lower temperatures • E is favored at higher temperatures
Recommend
More recommend