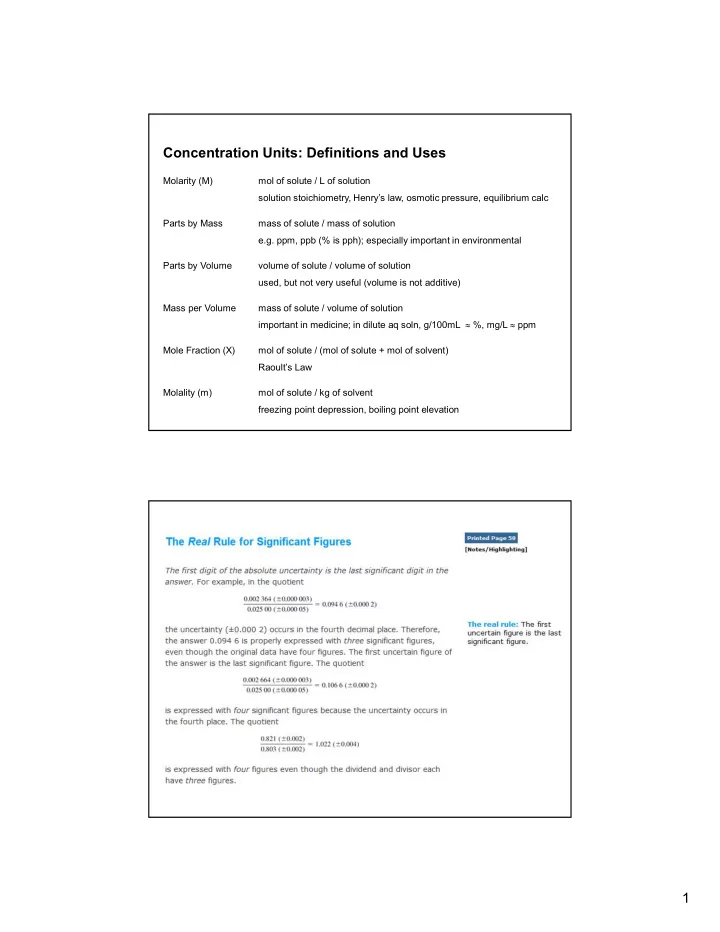

Concentration Units: Definitions and Uses Molarity (M) mol of solute / L of solution solution stoichiometry, Henry’s law, osmotic pressure, equilibrium calc Parts by Mass mass of solute / mass of solution e.g. ppm, ppb (% is pph); especially important in environmental Parts by Volume volume of solute / volume of solution used, but not very useful (volume is not additive) Mass per Volume mass of solute / volume of solution important in medicine; in dilute aq soln, g/100mL %, mg/L ppm Mole Fraction (X) mol of solute / (mol of solute + mol of solvent) Raoult’s Law Molality (m) mol of solute / kg of solvent freezing point depression, boiling point elevation 1
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spect rpy/nmr/nmr1.htm 2
http://scratch.mit.edu/projects/traincode/705416 3
https://www.math10.com/en/algebra/probabilities/binomial- theorem/binomial-theorem.html https://wonderopolis.org/wonder/what-is-pascals-triangle 4
45000 40000 35000 30000 25000 20000 15000 10000 5000 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 45000 40000 35000 30000 25000 20000 15000 10000 5000 0 1 2 3 4 5 6 7 8 9 1011121314151617 5
Defining a new dependent coordinate ( z ) in terms of x , the mean, and the standard deviation and plotting y versus z gives the graph on the right. (p. 70, Harris 8e) 6
Radial wavefunction for 1s orbit. Note how amount of electron density decreases in each band out from the nucleus but never reaches zero. https://www.chemistry.mcmaster.ca/esam/Chapter_3/section_2.html https://www.chemistry.mcmaster.ca/esam/Chapter_3/section_2.html 7
8
Three common cases for using the t test to compare measured values: 1. the mean of one sample is compared to a “known” value or established standard Example: molarity “known” to be 0.1147 M 2. the mean of one sample is compared to the mean of another sample Examples: concentration of phenol in Hogtown Creek; average height of CHM 3120 students 3. comparing individual data points in a set of paired measurements; this approach is commonly used to compare different analytical techniques (p. 78, Harris 8e) 9
Student Heights, CHM 3120, Spring 2019 16 14 12 Number of Students 10 8 6 4 2 0 57-58 59-60 61-62 63-64 65-66 67-68 69-70 71-72 73-74 75-76 Height (inches) 10
Three common cases for using the t test to compare measured values: 1. the mean of one sample is compared to a “known” value or established standard Example: molarity “known” to be 0.1147 M 2. the mean of one sample is compared to the mean of another sample Examples: concentration of phenol in Hogtown Creek; average height of CHM 3120 students 3. comparing individual data points in a set of paired measurements; this approach is commonly used to compare different analytical techniques (p. 78, Harris 8e) 11
175 mg/dL x (1 g/1000 mg) x (10 dL/1L) = 1.75 g/L 12
13
Rank the following solutions in order of increasing pH: 0.1 M HNO 2 , 0.1 M HNO 3 , 0.1 M NaNO 2 , 0.1 M NaNO 3 , 0.1 M NaOH A. HNO 2 < HNO 3 < NaNO 2 < NaNO 3 < NaOH B. HNO 3 < HNO 2 < NaNO 2 < NaNO 3 < NaOH C. HNO 2 < HNO 3 < NaNO 3 < NaNO 2 < NaOH D. HNO 3 < HNO 2 < NaNO 3 < NaNO 2 < NaOH E. HNO 3 < HNO 2 < NaOH < NaNO 3 < NaNO 2 14
15
14 12 10 8 pH HCl 6 HNO2 4 2 0 0 10 20 30 40 50 60 70 80 90 100 Volume of 0.10 M NaOH added (mL) 16
17
Rank the following solutions in order of increasing pH: 0.1 M HNO 2 , 0.1 M HNO 3 , 0.1 M NaNO 2 , 0.1 M NaNO 3 , 0.1 M NaOH A. HNO 2 < HNO 3 < NaNO 2 < NaNO 3 < NaOH B. HNO 3 < HNO 2 < NaNO 2 < NaNO 3 < NaOH C. HNO 2 < HNO 3 < NaNO 3 < NaNO 2 < NaOH D. HNO 3 < HNO 2 < NaNO 3 < NaNO 2 < NaOH E. HNO 3 < HNO 2 < NaOH < NaNO 3 < NaNO 2 18
19
20
A 50 mL aliquot of 0.10 M HNO 2 ( aq ) is titrated with 0.10 M NaOH(aq) ( aq ) + H 2 O( l ) HNO 2 ( aq ) + OH ( aq ) NO 2 The pK a of HNO 2 is 3.15. After 15 mL of OH (aq) is added, the pH will be… A. less than 3.15 B. 3.15 C. greater than 3.15, but less than 7 D. greater than 7, but less than 10.85 E. greater than 10.85 21
A 50 mL aliquot of 0.10 M HNO 2 ( aq ) is titrated with 0.10 M NaOH(aq) ( aq ) + H 2 O( l ) HNO 2 ( aq ) + OH ( aq ) NO 2 The pK a of HNO 2 is 3.15. After 70 mL of OH (aq) is added, the pH will be… A. less than 13.00 B. 13.00 C. greater than 13.00 22
Recommend
More recommend