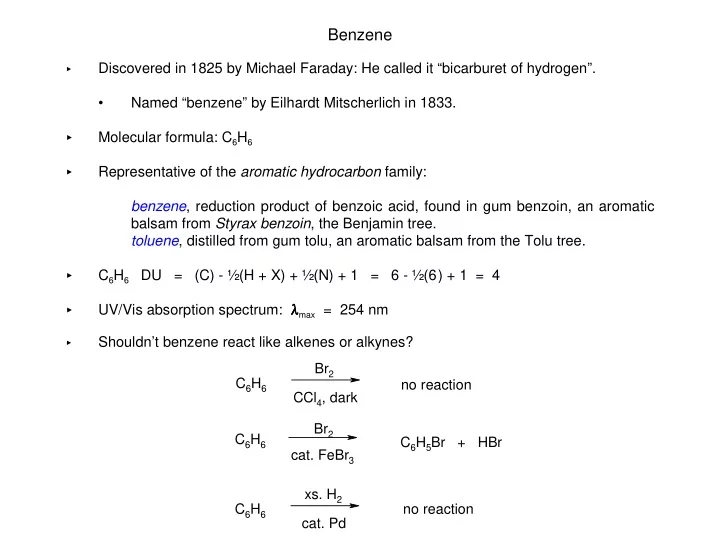

Benzene � Discovered in 1825 by Michael Faraday: He called it “bicarburet of hydrogen”. • Named “benzene” by Eilhardt Mitscherlich in 1833. � Molecular formula: C 6 H 6 Representative of the aromatic hydrocarbon family: � benzene , reduction product of benzoic acid, found in gum benzoin, an aromatic balsam from Styrax benzoin , the Benjamin tree. toluene , distilled from gum tolu, an aromatic balsam from the Tolu tree. � C 6 H 6 DU = (C) - ½(H + X) + ½(N) + 1 = 6 - ½(6) + 1 = 4 UV/Vis absorption spectrum: � � � � max = 254 nm � � Shouldn’t benzene react like alkenes or alkynes? Br 2 C 6 H 6 no reaction CCl 4 , dark Br 2 C 6 H 6 C 6 H 5 Br + HBr cat. FeBr 3 xs. H 2 C 6 H 6 no reaction cat. Pd
Some Possible Structures for Benzene C 6 H 6 DU = 4 Dewar benzene: H H H Dewar, James, Proceedings of the Royal Society of Edinburgh 1866/1867 , 6 , 82. H H H H H Ladenburg benzene: H Ladenburg, Albert, Chemische Berichte 1869 , 2 , 140. H H H Loschmidt’s C 6 = one hexavalent C concept: Loschmidt, Joseph Konstitutions Formeln der organischen Chemie in graphischer Darstellung, Chemische Studien I , Vienna, 1861.
Kekulé Benzene C 6 H 6 DU = 4 H H H H H H H H H H H H H H H H H H “ I was sitting writing at my textbook but the work did not progress; my thoughts were elsewhere. I turned by chair to the fire and dozed. Again the atoms were gamboling before my eyes. . .My mental eye. . .could now distinguish larger structures of manifold conformation: long rows. . .all twining and twisting in snake-line motion. But look! What was that? One of the snakes had seized hold of its own tail, and the form whirled mockingly before my eyes. . .I awoke; and this time also I spent the rest of the night in working out the consequences of the hypothesis. Let us learn to dream, gentlemen, then perhaps we shall find the truth. But let us beware of publishing our dreams till they have been tested by the waking understanding.” (Rapp, J. “Kekulé Memorial Lecture,” Journal of the Chemical Society 1898 , 73 , 100.)
H H H H H H H H H H H H C 6 H 6 Benzene X-ray crystallographic studies show that: � all C–C bonds are the same length, 1.397 � all � CCC are equivalent, 120 � (therefore, all carbons are sp 2 -hybridized) � � recall: C sp 2 –C sp 2 σ bond = 1.46 � C sp 2 =C sp 2 σ + π bond = 1.34 � � so, benzene’s carbon-carbon bonds are like the bonds of conjugated alkadienes, with partial double bond character.
LCAO diagrams of benzene sigma bond system pi bond system
Tremendous resonance stabilization : 1,3,5-cylcohexatriene, a hypothetical molecule, vs. benzene, a real molecule: 86 H 2 Pt /C � ( � H) = 36 kcal / mol 58 � ( � H) = 4 kcal / mol 54 54 E H 2 Pt /C 50 kcal / mol H 2 Pt /C H 2 Pt /C 3 H 2 Pt /C 28 28 28 H 2 Pt /C H 2 Pt /C H 2 Pt /C
Nomenclature of Arenes name as a substituted benzene: H F ( S )- sec -butylbenzene fluorobenzene or ( S )-(1-methylpropyl)benzene NO 2 nitrobenzene
Common Arenes: toluene styrene 1,2-dimethylbenzene ortho -xylene o -xylene O OH H phenol benzaldehyde 1,3-dimethylbenzene meta -xylene m -xylene O O NH 2 NH 2 OH OH aniline benzoic acid 1,4-dimethylbenzene para -xylene p -xylene
Three examples of multiply substituted arenes 1-bromo-4-methylbenzene Br 4-bromotoluene p -bromotoluene ( ortho , meta , and para are used only for disubstituted benzenes) 2-ethyl-1-isopropyl-4-methylbenzene 3-ethyl-4-isopropyltoluene NO 2 2,4,6-trinitrotoluene (TNT) O 2 N NO 2
Common substituents containing aromatic rings , C 6 H 5 – “phenyl” trans -2-phenyl-1-cyclohexanol OH , C 6 H 5 CH 2 – “benzyl” cis -4-benzyl-3-bromo-1-cyclopentene Br
Do all all- sp 2 hybridized ring systems exhibit aromaticity? 1,3-cyclobutadiene benzene (1Z,3Z,5Z,7Z)1,3,5,7-cyclooctaetraene 4 � electrons 6 � electrons 8 � electrons C2-C3 and C4-C1 planar nonplanar, so p orbtials don’t overlap all p orbtials overlap p orbtials don’t overlap not aromatic aromatic not aromatic
Is (1 E , 3 Z , 5 E , 7 Z , 9 Z )-cyclodecapentaene an aromatic hydrocarbon? H H H H H H H H H H
Aromaticity of Heterocycles H H H H H N H N H H H H H H H H H .. H H N H N H .. H pyrrole pyridine 6 π electrons in 5 p orbitals 6 π electrons in 6 p orbitals
Aromaticity of Heterocycles H H H H N O O H H H H H H H H N .. .. .. H H O O .. .. furan oxazoline not aromatic: one of the ring 6 π electrons in 5 p orbitals carbons is sp 3 -hybridized
Spectroscopy of aromatic compounds • IR aromatic overtones 95 90 85 ~ 1600 C sp 2 =C sp 2 80 stretch 75 70 > 3000 C sp 2 –H stretch 65 %Transmittance 60 55 50 45 40 35 30 25 20 ~ 900 - 680 C sp 2 –H bend 15 10 4000 3500 3000 2500 2000 1500 1000 500 Wavenumbers (cm-1)
Spectroscopy of aromatic compounds • NMR benzylic hydrogens: ~ 2.2 - 3.0 ppm aromatic hydrogens: ~ 6.5 - 8.0 ppm
Spectroscopy of aromatic compounds • NMR CH CH aromatic C region: ~ 110 - 170 ppm CH 3 C
Spectroscopy of aromatic compounds • MS allkylbenzenes often have a prominent m/ z = 91, the benzyl or tropilium ion + + or
Spectroscopy of aromatic compounds • UV-Vis arenes typically have”B band” λ max in the region 254 - 280 nm; occasionally may also see the more intense “E band” at shorter λ . λ max = 265 nm
Recommend
More recommend