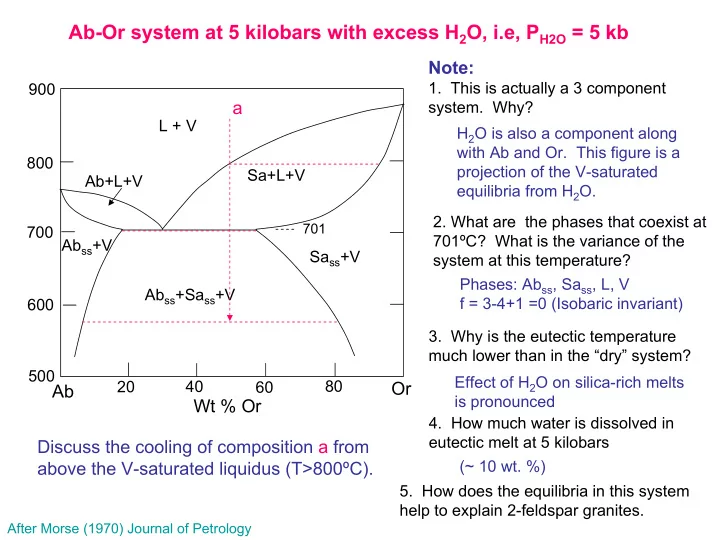

Ab-Or system at 5 kilobars with excess H 2 O, i.e, P H2O = 5 kb Note: 1. This is actually a 3 component 900 a system. Why? L + V H 2 O is also a component along with Ab and Or. This figure is a 800 projection of the V-saturated Sa+L+V Ab+L+V equilibria from H 2 O. 2. What are the phases that coexist at 701 700 701ºC? What is the variance of the Ab ss +V Sa ss +V system at this temperature? Phases: Ab ss , Sa ss , L, V Ab ss +Sa ss +V f = 3-4+1 =0 (Isobaric invariant) 600 3. Why is the eutectic temperature much lower than in the “dry” system? 500 Effect of H 2 O on silica-rich melts 20 40 80 60 Or Ab is pronounced Wt % Or 4. How much water is dissolved in eutectic melt at 5 kilobars Discuss the cooling of composition a from (~ 10 wt. %) above the V-saturated liquidus (T>800ºC). 5. How does the equilibria in this system help to explain 2-feldspar granites. After Morse (1970) Journal of Petrology
c (001) a perthite TEM image of albite lamellae (showing polysynthetic twinning) in a K-feldspar host (100) cleavage Plane // (010) Sketch showing the orientation of perthitic lamellae of albite in a K- feldspar host Optical photomicrograph of wispy albite lamellae in a microcline host
Albite-H 2 O: Binary system with H 2 O to 1.0 Gpa (10 kb) Phase rule: Along V-saturated melting curve, c=2, p=?, f = ? What is not obvious from this figure is that the amount of H 2 O dissolved in albite melt at vapor-saturation increases dramatically along the the vapor-saturated curve (shown below and on next figure). P (kb) Wt% H 2 O Mol % H 2 O 1 4.4 40 2 6.4 50 4 8.7 58 This P-T projection shows two lines (a) dry melting of albite and 6 11.1 64 8 13.4 69 (b) melting of albite under water-saturated conditions (studied 10 15.8 73 by numerous experimentalists). Note: pronounced lowering of melting point with addition of water to the system. Why is there a dramatic change in slope of V-sat d melting curve? Figure from Winter (2001) An Introduction to Igneous and Metamorphic Petrology
Albite – H 2 O system (cont.) As discussed in the previous figure, it takes a lot of water to saturate a melt of albite composition (or any silicate melt for that matter). Chances are that there is not enough water in the melting region to saturate the melt under all conditions. What then? We can use the Ab-H 2 O system to illustrate this situation. Red curves: melting for fixed mol fr. H 2 O in melt Blue curves: water content of a H 2 O-sat d melt Let’s examine the melting path of comp n a at c b P= 5 kb with 50 mol% (6.4 wt %) H 2 O a At b: system begins to melt. System is isobaric invariant so it must remain at b until one of the phases is gone. Which one? Note that albite can dissolve 10 wt % H 2 O at 5 kb but we only have 6.4%. All H 2 O available is dissolved in melt and we are left with melt and some albite crystals From b to c, T increases and the albite continues to melt (no V phase in the system). When is melting complete? Melting is complete at c. Can show in (TX) P figure on next slide Figure from: Burnham and Davis (1974) AJS, 274
Albite - H 2 O system (cont.) Isobaric TX section PH 2 O = 5 kb V 1400 L 1200 T(ºC) L + V c b 1000 a c Ab+L 800 b 770 a Ab + V 600 80 20 40 60 H 2 O Ab Wt % H 2 O Figure from: Burnham and Davis (1974) AJS, 274
Albite-H 2 O system (Cont.) Isothermal decompression from 10 Kb to the surface starting at a (950ºC). Assume a contains 50 mol% (6.4 wt %) H 2 O. a What is the initial state of the system? Since albite melt at 950ºC & 10kb can hold b 52% H 2 O at sat n , the system will consist of 96% melt containing 52% H 2 O + 4% albite crystals giving a bulk comp n of 50% H 2 O. What happens at b as melt decompresses? All crystals melt, leaving melt with 50% H 2 O What happens between b and c? Melt becomes superheated (no V phase) What happens at c? Melt starts to outgas and vesiculate What happens between c and d? Melt continues to outgas and vesiculate What happens at d? c At d, melt contains ~30 mol% H 2 O and crystallization begins. Melt stays at d simultaneously outgassing and d crystallizing until totally crystallized. Do real magmas behave like this? Yes!! Figure from: Burnham and Davis (1974) AJS, 274
Albite-H 2 O system (cont.) What happens if the fluid phase is a mixture of species? In nature the fluid phase is likely to be a mixture of H 2 O, CO 2 , SO 2 along with other gaseous species. This figure shows the effect of “diluting” the fluid phase with addition of CO 2 . The activity of H 2 O is reduced below 1 and consequently its effect in lowering the melting temperature is reduced. Figure from Winter (2001) An Introduction to Igneous and Metamorphic Petrology.
Ternary (c = 3) systems This diagram is an attempt to show a Anorthite 3D diagram in a 2D perspective. The red lines show the binary eutectic between anorthite and forsterite, the blue lines show the eutectic between forsterite and diopside and the green a lines show the eutectic between diopside and anorthite. c Black lines extending from each of the binary eutectics extend into the 3D object and meet at the ternary T TE eutectic labeled TE. The black b lines are called cotectics . TE is the lowest temperature in the system and it can be compared to a hole where three valleys meet. An isothermal plane (not shown) Forsterite parallel to the base passing through Diopside TE is the ternary solidus plane. This system which has no solid solution is the simplest ternary system To depict this system in 2D, the liquidus surface is projected parallel to the T axis on to the base. This diagram can then be contoured with isotherms much like elevation contours on a topo map After: Winter (2001) An Introduction to Igneous and Metamorphic Petrology.
Ternary (c = 3) systems: Fo-An-Di system The liquidus surface projected onto the base of the triangle with the position of the ternary cotectic lines shown in orange. Fine lines are P = 1 atm isothermal contours on the liquidus surface. The ternary euctectic is at M (1270ºC); this is lowest temperature at which liquid can exist in this system. The orange lines are also called field boundaries since they delineate distinct fields of crystallization The composition of M can be read off the ternary figure as Di 50 -An 43 -Fo 7 . Phase rule at M: C = 3, p= ?, f = ? After: Morse (1994) Basalts and phase diagrams. Krieger
Fo-Di-An system at P = 1 atm Eq m crystallization of composition a: Fo crystallizes at 1500ºC and liquid follows path directly away from Fo corner to b (mass balance). At b, An joins Fo and both crystallize in ratio given by the tangent to the cotectic (30Fo + 70An) and the liquid follows the cotectic to M. At M, diopside joins the crystallization sequence and the three crystalline phases form in the relative amounts corresponding to composition M. Fractional crystallization follows the b same path, the only difference being that the crystals are removed from the system as they form. a Equilibrium melting is the reverse of eq m crystallization. The first liquid to form from any ratio of An:Fo:Di is at M. After: Morse (1994) Basalts and phase diagrams. Krieger
Illustration of the ternary lever rule x x a a Di Di m m Fo Fo T =1300ºC: Bulk composition = a liquid x crystallizing Di and Fo Ratio of xals/liq = ax/am Ratio of Di/Fo = Fo-m/Di-m After: Winter (2001) An Introduction to After: Morse (1994) Basalts and phase diagrams. Krieger Igneous and Metamorphic Petrology.
Ternary system (Fo-An-SiO 2 ) with intermediate compound (peritectic) Phase rule: At points c and d: f = ? Reaction along line labeled “peritectic”: Fo + L ↔ En All other field boundaries are cotectics, e.g., from 1543 to d, reaction is: L ↔ En + Silica After: Morse (1994) Basalts and phase diagrams. Krieger
Recommend
More recommend