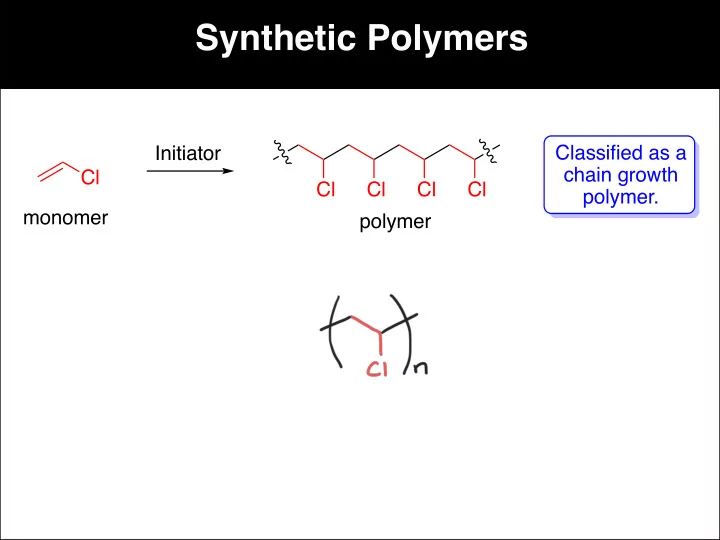

Synthetic Polymers Classified as a Initiator chain growth Cl Cl Cl Cl Cl polymer. monomer polymer
Synthetic Polymers The initiator can be a radical, a cation, or an anion. Mechanism for Radical Polymerization: Ph H 2 O 2 polystyrene Ph (styrofoam) n
Synthetic Polymers Anionic Polymerization takes place when there is an electron withdrawing group on the alkene. O CN etc. , ,
Synthetic Polymers Cationic Polymerization takes place when there is an electron donating group on the alkene. OCH 3 NH 2 etc. , ,
Synthetic Polymers Identifying the monomer used to make the polymer O O
Synthetic Polymers Ring Opening Polymerization O + HO
Recommend
More recommend