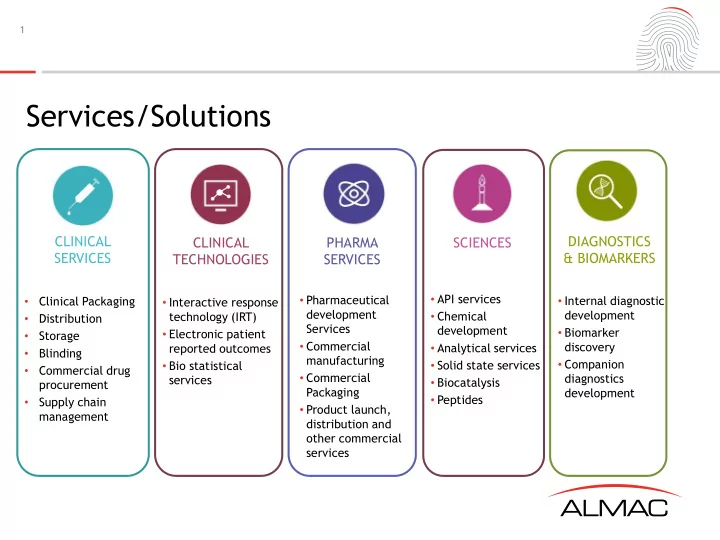

1 Services/Solutions CLINICAL DIAGNOSTICS CLINICAL PHARMA SCIENCES SERVICES & BIOMARKERS TECHNOLOGIES SERVICES • API services • Pharmaceutical • Internal diagnostic • Clinical Packaging • Interactive response development development technology (IRT) • Chemical • Distribution Services development • Biomarker • Electronic patient Storage • • Commercial discovery • Analytical services reported outcomes • Blinding manufacturing • Companion • Bio statistical • Solid state services • Commercial drug • Commercial diagnostics services • Biocatalysis procurement Packaging development • Peptides • Supply chain • Product launch, management distribution and other commercial services
2 Services/Solutions SCIENCES • API services • Chemical development • Analytical services • Solid state services • Biocatalysis • Peptides
3
4 Peptide Capabilities Non-GMP GMP Analytical Pre- custom Radiolabelling manufacture support formulation synthesis
5 Chemistry capabilities Lab nonGMP Kilolab nonGMP Plant GMP Pre-clinical Early Phase Late Phase Commercial
6 Chemistry capabilities Lab nonGMP Kilolab nonGMP Plant GMP Pre-clinical Early Phase Late Phase Commercial
7 Chemistry capabilities Lab nonGMP Kilolab nonGMP Plant GMP Pre-clinical Early Phase Late Phase Commercial
8 Solid State Services Drug Solid Drug substance State Product
9 Analytical services Drug substance Standalone Drug product Analytical labs at both UK and US sites
10 Services/Solutions CLINICAL DIAGNOSTICS CLINICAL PHARMA SCIENCES SERVICES & BIOMARKERS TECHNOLOGIES SERVICES • API services • Pharmaceutical • Internal diagnostic • Clinical Packaging • Interactive response development development technology (IRT) • Chemical • Distribution Services development • Biomarker • Electronic patient Storage • • Commercial discovery • Analytical services reported outcomes • Blinding manufacturing • Companion • Bio statistical • Solid state services • Commercial drug • Commercial diagnostics services • Biocatalysis procurement Packaging development • Peptides • Supply chain • Product launch, management distribution and other commercial services
11 Services/Solutions PHARMA SERVICES • Pharmaceutical development Services • Commercial manufacturing • Commercial Packaging • Product launch, distribution and other commercial services
12 Pharmaceutical Development Services 90-100 40 Analytical Formulation Staff Staff 20 Project Management Staff
13 Early Stage Development • Development of solid oral dose presentations Manufacturing of prototype: • Blends and Granulates • Tablets • Formulated Capsules and Drug in Capsule • Powder in Sachet • • API in bottle & oral liquid formulations • Clinical supply phase I (First in Man Trials) First in man trials in health patients • Small batch GMP manufacture • Shipment to trial site or client •
14 Scale-up Development Development of immediate release oral dose formulations • Tablets, minitabs and sublingual tablets • Capsules • Powders for sachet • Development of modified release oral dose formulations • Swellable matrix • Delayed release coatings • Permeable coatings • Coated pellets • Enteric coated Tablets and pellets • Clinical Supply for Phase IIa/IIb • Larger trials looking a dose tolerability in healthy patients • Larger batch manufacture •
15 Early Stage Development Later Stage Development Further formulation development, scale up, & process • investigations Clinical Supply for Phase III and Registration Batches • Very large multisite trials looking at effectiveness of treatment • Large batch manufacture (> 1 million dosage units) • 1 st Commercial batches manufacture •
16 Commercial Solid Oral Dose Manufacturing Dry Blending Fluid Bed Drying • Up to 2,500Kg • Up to 250Kg Compression Encapsulation • Up to 300,000 • Up to 100,000 tablets/hr capsules/hr Coating • Up to 300Kg
17 Commercial Solid Oral Dose Manufacturing 55 Commercial clients • • Processing 75 commercial products Large scale commercial Standard supply: commercial supply: • >400 million sachets / annum • 500Kg blend = Niche / 10-20 million orphan drugs: tablets per batch • 5k vials / annum
18 Commercial Solid Oral Dose Packaging Process Equipment Capacity Compression ~ 6.2 billion tablets Encapsulation ~ 1 billion capsules Blister packaging ~ 565 million blisters Bottle packaging ~ 52 million bottles Sachet packaging ~ 1 billion sachets Stickpack ~ 10 million stickpacks Wallet packaging ~ 41 million wallets Vial / ampoule labelling ~ 146 million units Syringe labelling ~ 20 million units *24 / 7, 45wks p.a.
19 Commercial Product Launch Commercial Supply Pre-submission Submission • • Artwork • QP Declaration & Product Ordering • • Shipment Site of Release Serialization Validation • • Packaging Design Product • EU Import Testing Distribution to Pharmacy
Recommend
More recommend