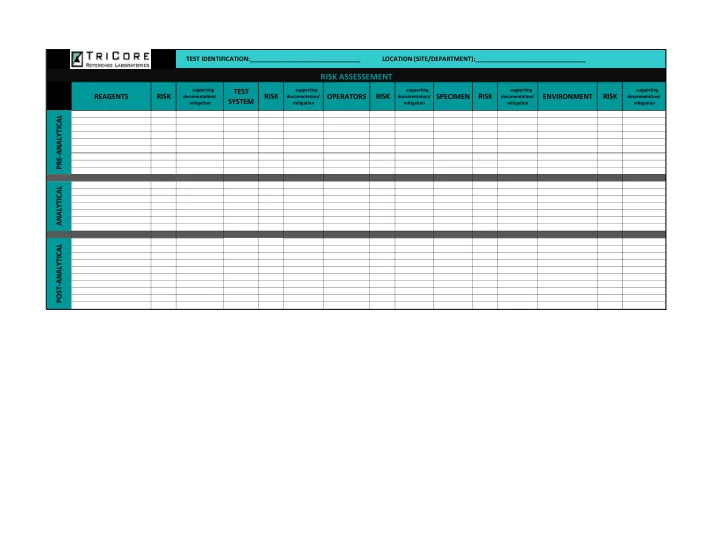

TEST IDENTIFICATION:________________________________ LOCATION (SITE/DEPARTMENT):________________________________ RISK ASSESSEMENT supporting TEST supporting supporting supporting supporting REAGENTS RISK RISK OPERATORS RISK SPECIMEN RISK ENVIRONMENT RISK documentation/ documentation/ documentation/ documentation/ documentation/ SYSTEM mitigation mitigation mitigation mitigation mitigation PRE-ANALYTICAL ANALYTICAL POST-ANALYTICAL
TEST IDENTIFICATION:_______________________________________ LOCATION (SITE/DEPARTMENT):_______________________ QUALITY CONTROL PLAN FREQUENCY CRITERIA FOR ACCEPTABILITY TYPE OF QUALITY CONTROL (specify the number, type and frequency of testing QC (Range of Acceptable Values) materials) SPECIMEN PRE ANALYTICAL TEST SYSTEM REAGENT ENVIRONMENT TESTING PERSONNEL SPECIMEN TEST SYSTEM ANALYTICAL REAGENT ENVIRONMENT TESTING PERSONNEL SPECIMEN POST ANALYTICAL TEST SYSTEM REAGENT ENVIRONMENT TESTING PERSONNEL Date: Medical Director Signature:
TEST IDENTIFICATION:_______________________________________ LOCATION (SITE/DEPARTMENT):_______________________ QUALITY ASSESSMENT PLAN ASSESSMENT OF QA QUALITY ASSESSMENT ACTIVITY CORRECTIVE ACTION FREQUENCY ACTIVITY (to monitor) (when indicated) (of monitoring) (Was there variation from established policy and procedures?) SPECIMEN PRE ANALYTICAL TEST SYSTEM REAGENT ENVIRONMENT TESTING PERSONNEL SPECIMEN TEST SYSTEM ANALYTICAL REAGENT ENVIRONMENT TESTING PERSONNEL SPECIMEN POST ANALYTICAL TEST SYSTEM REAGENT ENVIRONMENT TESTING PERSONNEL
Recommend
More recommend