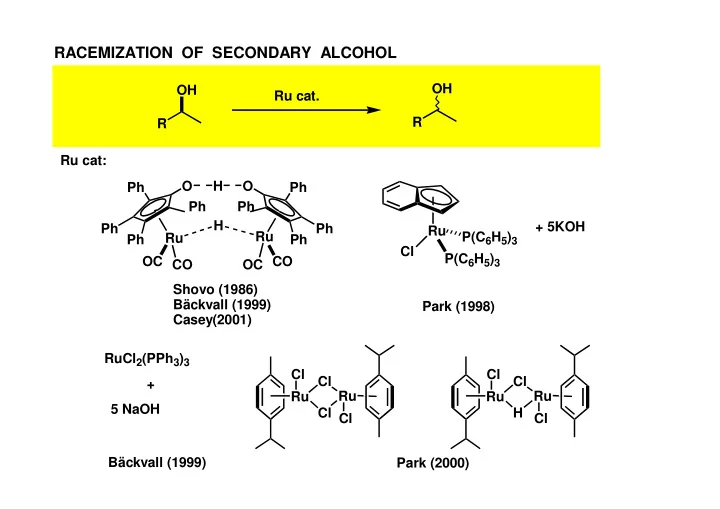

RACEMIZATION OF SECONDARY ALCOHOL OH OH Ru cat. R R Ru cat: O H O Ph Ph Ph Ph H + 5KOH Ph Ph Ru Ru P(C 6 H 5 ) 3 Ru Ph Ph Cl P(C 6 H 5 ) 3 OC CO CO OC Shovo (1986) Bäckvall (1999) Park (1998) Casey(2001) RuCl 2 (PPh 3 ) 3 Cl Cl Cl Cl + Ru Ru Ru Ru 5 NaOH Cl H Cl Cl Bäckvall (1999) Park (2000)
DEHYDROGENATIVE TRANSFORMATION OF SECONDARY ALCOHOL Cp*RuCl(cod) OH OH ligand KOH Ru C 6 H 5 C 6 H 5 2-propanol HN P(C 6 H 5 ) 2 >99% ee 50°C, 30 min 日本 日 本化 化学 学会 会第 第7 78 8春 春季 季年 年会 会 , 1F514 (2000) 日 日 本 本 化 化 学 学 会 会 第 第 7 7 8 8 春 春 季 季 年 年 会 会 ligand (C 6 H 5 ) 2 P NH 2 (C 6 H 5 ) 2 P NH(CH 3 ) (C 6 H 5 ) 2 P N(CH 3 ) 2 0% ee 9% ee 98% ee P(C 6 H 5 ) 3 ( 1 eq. ) (C 6 H 5 ) 2 P P(C 6 H 5 ) 2 (H 3 C) 2 N NH 2 98% ee 98% ee 98% ee
DEHYDROGENATIVE TRANSFORMATION OF ALCOHOLS Ar Ar Ar Ar P P Ru Ru H H N N H H R' R' C C H H O O R R EPIMERIZATION OF meso -DIOLS ISOMERIZATION OF ALLYLIC ALCOHOLS LACTONIZATION OF α α α , ω α ω ω ω -DIOLS
ENANTIOMER DISCRIMINATION OF SECONDARY ALCOHOL Cp*RuCl(cod) H OH OH chiral ligand N P(C 6 H 5 ) 2 KOH * S R R 2-propanol, 30 °C R = C 6 H 5 or n -C 4 H 9 chiral ligand alcohol:Ru:chiral ligand:KOH = 100:1:1.5:1 amide complex R = C 6 H 5 k S /k R = 1.3 R = n -C 4 H 9 k S /k R = 1.8 Ru N P(C 6 H 5 ) 2
ENANTIOMER DISCRIMINATION OF sym -DIOL Cp*RuCl(cod) OH OH chiral ligand H C 6 H 5 KOH C 6 H 5 S N P(C 6 H 5 ) 2 C 6 H 5 C 6 H 5 R S OH 2-propanol, 30 °C OH chiral ligand meso dl hydrobenzoin:Ru:chiral ligand:KOH = 100:1:1.5:1 100 80 conversion, % 60 40 R,R -diol 20 S,S -diol 0 2 4 6 8 time, h
EPIMERIZATION OF meso -HYDROBENZOIN WITH ACHIRAL LIGAND Cp*RuCl(cod) OH ligand OH H 2 N P(C 6 H 5 ) 2 KOH C 6 H 5 C 6 H 5 S * C 6 H 5 * C 6 H 5 R 2-propanol, 30 °C OH OH ligand meso dl hydrobenzoin:Ru:ligand:KOH = 100:1:1.5:1 100 80 conversion, % 60 40 20 0 10 20 reaction time, h
EPIMERIZATION OF meso -HYDROBENZOIN WITH CHIRAL LIGAND Cp*RuCl(cod) OH OH chiral ligand H N P(C 6 H 5 ) 2 C 6 H 5 C 6 H 5 R S KOH S C 6 H 5 C 6 H 5 R R 2-propanol, 30 °C OH OH meso chiral ligand dl hydrobenzoin:Ru:chiral ligand:KOH = 100:1:1.5:1 100 ee conversion and ee, % 80 60 conv. 40 20 0 2 4 6 8 10 time, h
SYNTHESIS OF OPTICALLY ACTIVE DIOLs FROM meso -DIOLs Cp*RuCl(cod) OH OH chiral ligand KOH R R S R R R R R OH OH 2-propanol, 30 °C, 4 h meso -diol:Ru:chiral ligand:KOH = 100:1:1.5:1 conc. conv, % ee, % R C 6 H 5 49 99 0.1 M H p -CH 3 -C 6 H 4 0.05 M 16 91 N P(C 6 H 5 ) 2 S p -F-C 6 H 4 0.1 M 22 85 CH 3 0.1 M 18 98 chiral ligand –(CH 2 ) 4 –� - 0.1 M 0
A POSSIBLE MECHANISM FOR EPIMERIZATION OH OH C 6 H 5 R C 6 H 5 R C 6 H 5 C 6 H 5 R OH O R,R OH R C 6 H 5 S C 6 H 5 R OH OH O C 6 H 5 S meso C 6 H 5 C 6 H 5 S S C 6 H 5 OH OH S,S S dl O O KOH C 6 H 5 C 6 H 5 S 2-propanol C 6 H 5 C 6 H 5 30 °C, 1h OH OH S/C = 100
SUMMARY Epimerization of diols Cp*RuCl(cod) OH OH chiral ligand R R KOH R S R R R R OH OH 2-propanol, 30 °C chiral ligand: H N P(C 6 H 5 ) 2 S
Recommend
More recommend