
Protein Folding
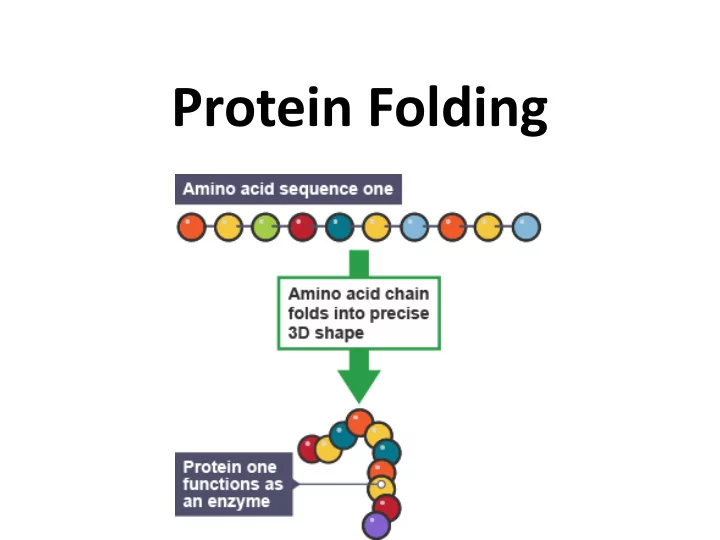
Protein Folding • Proteins have unique 3-dimensional shapes created by the twisting or folding of one or more polypeptide chains • The structure of a protein enables it to recognize and bind to other molecules
Protein Folding
Shape Determines Function
Shape Determines Function
Shape Determines Function
Protein Structure • Primary Structure • Secondary Structure • Tertiary Structure • Quaternary Structure
Primary Structure • Sequence of a chain of amino acids held together by peptide bonds
Secondary Structure • Involves regions of coiling or folding of the polypeptide • Stabilized by hydrogen bonds between the carboxyl and amino groups of amino acids
Tertiary Structure • Results from interactions between the various side chains (R groups) • Ionic bonds between positive (+) and negative (-) side chains • Hydrophobic interactions between nonpolar side chains (repelled by water), clump together • Hydrogen bonds between polar side chains • Disulfide bridges between the sulfhydryl groups of cysteine amino acids
Tertiary Structure
Quaternary Structure • Occurs in proteins that are composed of more than one polypeptide • Results from the combination of hydrogen bonding, ionic bonding, and hydrophobic interactions between polypeptide chains
Sickle-Cell Anemia Mutation • Causes hemoglobin molecules to crystallize when oxygen levels are low
Insulin Receptor
Recommend
More recommend