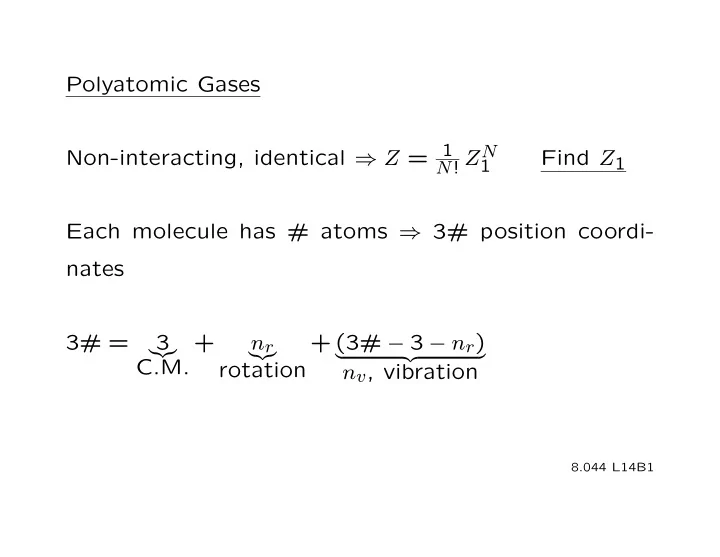

Polyatomic Gases Non-interacting, identical ⇒ Z = 1 Z N Find Z 1 1 N ! Each molecule has # atoms ⇒ 3# position coordi- nates 3# = ���� + 3 n r + (3# − 3 − n r ) ���� � �� � C.M. rotation n v , vibration 8.044 L 1 4B1
MONATOMIC DIATOMIC LINEAR TRI. NON-LINEAR TRI. Xe HS CO 2 H 2 O 3 3 3 3 0 2 2 3 0 1 4 3 3 6 9 9 8.044 L14B2
C.M. Motion: Particle in a box ∆ E s ≪ kT ⇒ classical Rotation: 12 (H 2 ν rot = 3 65 × 10 . Hz → 175 K ) ⇒ Q.M. Vibration: 14 (H 2 ν vib = 1 32 × 10 . Hz → 6 , 320 K ) ⇒ Q.M. H = H CM + H vib + H rot ⇒ problem separates 8.044 L 1 4B3
Vibration n v 1 1 K i ˙ 2 2 H vib = � K i a i + a i 2 2 2 ω i i =1 n v 1 dimensional harmonic oscillators, use Q.M. ǫ n = ( n + 1 )¯ ˆ H ψ n = ǫ n ψ n hω n = 0 1 , 2 , , · · · 2 The energy levels are non-degenerate. 8.044 L 1 4B4
1 hω/kT ∞ − ( n + )¯ ǫ /kT e − n � p n ) = e ( / 2 ε n =0 h ω n ∞ ∞ − ( n + 1 1 � � )¯ ¯ hω/k T e − hω/kT − hω ¯ /kT � � e = e 2 2 n =0 n =0 7 h ω 1 2 5 h ω − 1 1 � � ¯ hω/kT ¯ − hω/kT 2 = / 1 − e e 2 3 2 h ω 1 1 2 h ω 1 n � � � � e − hω/kT ¯ ¯ hω /kT = (1 − b ) n e − p ( n ) = 1 − b 8.044 L 1 4B5
Geometric or Bose-Einstein p(n) n b 1 < n > = = e hω/kT − 1 ¯ 1 − b → e − ¯ hω/kT when kT ≪ ¯ hω 8.044 L 1 4B6
1 For kT ≫ ¯ hω < n > → 2 hω + 1 � � 1 + ¯ ¯ hω · · · − 1 2 kT kT kT 1 kT � ¯ hω � �� − 1 = ¯ 1 ≈ 2 � � hω 1 + 1 ¯ hω ¯ hω kT 2 kT kT hω − 1 = ¯ 2 < ǫ > = ( < n > + 1 2 )¯ kT ≫ ¯ hω (Classical) hω → kT 1 ¯ ≪ ¯ hω (Ground state) hω kT → 2 8.044 L 1 4B7
<n> < ε > 3 2 kT 1 1 h ω 2 0 T 1 2 3 kT/h ω -1 8.044 L14B8
� ∂ < ǫ > d < n > � C V = N = N ¯ hω ∂T dT V � 2 ¯ e hω/kT � ¯ hω = Nk � ¯ � 2 hω/kT − 1 kT e � 2 � ¯ hω e − ¯ hω/kT → Nk kT ≪ ¯ hω ( energy gap behavior ) kT → Nk kT ≫ ¯ hω 8.044 L 1 4B9a
C V /Nk 1 ENERGY GAP BEHAVIOR 2 LEVEL SYSTEM SHOWING SATURATION 1 2 3 kT/h ω 8.044 L14B9b
High and low temperature behavior without solving the complete problem Consider first the high T limit. e - ε /kT ∆ ǫ contains ∆ ǫ hω states ¯ kT ε h ω ∞ e − ǫ n /k T � Z 1 = n =0 � ∞ 1 ∞ e − y kT kT � E/kT hω ∝ β − 1 − e dE = dy = ≈ 0 hω 0 ¯ hω ¯ ¯ 8.044 L 1 4B10
Z vib = Z 1 ∝ β − N N 1 ∂Z = − N N 1 β − − U vib = − ( − N ) = NkT β Z ∂β N C vib = Nk Next, consider the low T limit. e - ε /kT ⇒ consider only 2 states kT 1 3 ε h ω 2 h ω 2 8.044 L 1 4B11
− 3 ¯ hω/kT e 1 2 ¯ hω/kT − p ( n = 1) ≈ = e ≈ e hω/kT + 1 1 3 ¯ ¯ hω/kT ¯ hω/kT e − 2 + e − 2 p ( n = 0) ≈ 1 − e − ¯ hω/kT � � − ¯ hω /k T ¯ hω /kT hωe − 1 + 3 < E > = 2 N ¯ hω 1 e 2 N ¯ − ¯ hω/kT hωe − = 1 2 N ¯ hω + N ¯ � ¯ 2 ∂ < E > hω � ¯ hω � � hω/kT = Nk − ¯ e − ¯ hω/kT C V = = N ¯ hω e 2 ∂T kT kT 8.044 L 1 4B12
Angular Momentum in 3 Dimensions , L ); ( | � CLASSICAL, 3 numbers: ( L x , L L | , θ, φ ) y z QUANTUM, 2 numbers: magnitude and 1 component ˆ ˆ L ψ l,m ≡ ˆ 2 L · � � L 2 ψ l,m = l ( l + 1)¯ = 0 1 , 2 · · · h ψ l,m l , ˆ z ψ l,m = m ¯ m = l, l − 1 , · · · − l L h ψ l,m � �� � 2 l +1 values 8.044 L 14 B1 3
Specification: 2 numbers l & m → or | l, m > ψ l,m Molecular rotation In general 3 = 0 I 1 1 1 2 2 2 H rot = L 1 + L 2 + L 3 ˙ 2 I 1 2 I 2 2 I 3 L 3 = I 3 θ 3 = 0 For a linear molecule 1 1 1 = I 2 I ≡ I ⊥ 2 2 � � H rot = ( L 1 + L 2 ) = L L · 2 I 2 I ⊥ ⊥ 8.044 L 14 B2 14
1 ˆ 2 ˆ H rot = L 2 I ⊥ ε/ k ˆ H rot | l, m > = ǫ l | l, m > l = 4 20 Θ R 9 ¯ 2 h = l ( l + 1) l, m > | 2 I ⊥ l = 3 12 Θ R 7 ǫ l depends on l only; it is 2 l + 1 fold degenerate. l = 2 6 Θ R 5 ǫ l = k Θ R l ( l + 1) l = 1 2 Θ R 3 0 l = 0 1 ¯ 2 h Θ R ≡ ( rotational temp. ) 2 I ⊥ k 8.044 L 14 B 15
1 e − l ( +1)Θ R /T l p l, m ) = ( Z R R /T = e − l ( l +1)Θ l + 1) e − l ( +1)Θ l R /T � � Z R = (2 l,m l 2Θ /T 2Θ kβ 1 + 3 e − = 1 + 3 e − For T ≪ Θ Z R R ≈ R R 2Θ R kβ 1 ∂Z 6Θ R k e − 2Θ R /T R k e − < ǫ > = − = 6Θ ≈ − 2Θ R kβ 1 + 3 e Z ∂β 8.044 L 14 B 16
∂ < ǫ > � 2Θ � R e − 2Θ R /T C V | rot = N = 6Θ R Nk T 2 ∂T � 2 � 2Θ R e − 2Θ R /T = 3 Nk ( energy gap behavior ) T For T ≫ Θ R , convert the sum to an integral. ∞ � l + 1) e − l l ( +1)Θ /T Z (2 R dl R ≈ 0 8.044 L 14 B 17
2 x ≡ ( l + l )Θ R /T dx = (2 l + 1)Θ R /T dl � ∞ T T 1 − x − 1 Z R ≈ e dx = = β Θ 0 Θ R k Θ R R 1 ∂Z ( − 1)( − 1) Z/β = β − 1 < ǫ > = − = = kT Z ∂β Z ∂ < ǫ > C V | rot = N → Nk ( classical result ) ∂T 8.044 L 14 B 18a
H = H CM + H rot + H vib C V ( T ) = C � V | + C | + C | CM � V rot � V vib �� � �� � �� � all T appears at modest T only at highest T 8.044 L 14 B 19 a
Raman Scattering BEFORE AFTER ε i ε f ν i ν f ∆ε = ε f - ε i = h ( ν i - ν f) FREQUENCY CHANGES IN THE SCATTERED LIGHT CORRESPOND TO ENERGY LEVEL DIFFERENCES IN THE SCATTERER. WHICH ENERGY LEVEL CHANGES OCCUR DEPEND ON SELECTION RULES GOVERNED BY SYMMETRY AND QUANTUM MECHANICS 8.044 L14B20
Example Rotational Raman Scattering Selection rule: ∆ l = ± 2 ∆ ν l = − ( k Θ R /h )[( l + 2)( l + 3) − l ( l + 1)] ↑ = − (4 l + 6)( k Θ R /h ) ⇒ uniform spacing between lines of 4( k Θ R /h ) I l ∝ number of molecules with angular momentum l ↑ ∝ (2 l + 1) e − l ( l +1)Θ R /T 8.044 L 14 B 21
ROTATIONAL RAMAN SPECTRUM OF A DIATOMIC MOLECULE I ( ∆ν ) BOLTZMANN FACTOR LEVEL DEGENERACY 0 ∆ν 4(k Θ R /h) -6(k Θ R /h) 8.044 L14B22
MIT OpenCourseWare http://ocw.mit.edu 8.044 Statistical Physics I Spring 2013 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
Recommend
More recommend