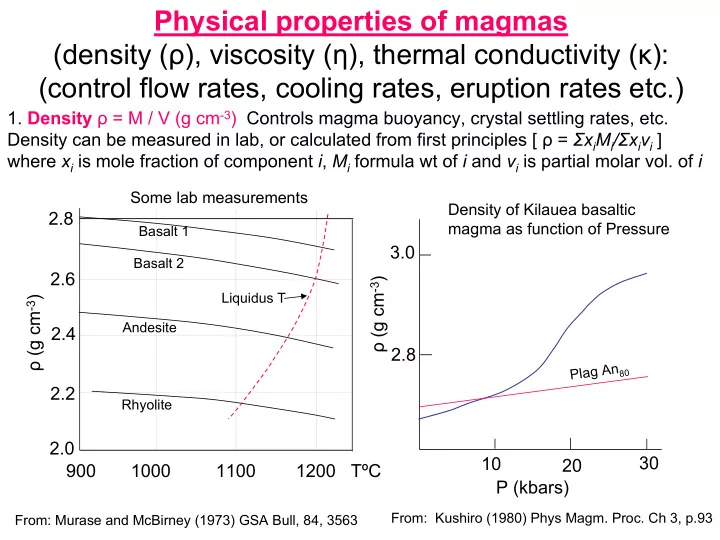

Physical properties of magmas (density ( ρ ), viscosity ( η ), thermal conductivity ( κ ): (control flow rates, cooling rates, eruption rates etc.) 1. Density ρ = M / V (g cm -3 ) Controls magma buoyancy, crystal settling rates, etc. Density can be measured in lab, or calculated from first principles [ ρ = Σ x i M i / Σ x i v i ] where x i is mole fraction of component i , M i formula wt of i and v i is partial molar vol. of i Some lab measurements Density of Kilauea basaltic 2.8 magma as function of Pressure Basalt 1 3.0 Basalt 2 2.6 ρ (g cm -3 ) Liquidus T ρ (g cm -3 ) Andesite 2.4 2.8 Plag An 80 2.2 Rhyolite 2.0 30 10 20 900 1000 1100 1200 TºC P (kbars) From: Kushiro (1980) Phys Magm. Proc. Ch 3, p.93 From: Murase and McBirney (1973) GSA Bull, 84, 3563
Viscosity ( η ): measure of a fluid’s resistance to flow σ (shear stress) (Pa) η = σ / ė 1 v 1 dv/dz = velocity gradient normal to v 2 2 v 3 Units: Pa s applied shear stress = strain rate ė (s -1 ) 3 v 4 4 η is called the coefficient of viscosity Fixed plate If η is constant over a range of σ , the fluid is said to exhibit Newtonian viscosity. Viscosity can be measured in field, in the lab, or calculated from first principles. ln η Slope = E*/R The temperature dependence of viscosity is given by the Arrhenius Equation: ln η = A + E*/RT (1/T (deg -1 ) where E* is the activation energy for viscous flow, R is the gas constant, and T is the temperature in ºK. A plot of ln η vs. 1/T is a straight line. Some typical values of viscosity of magmas are shown in the next slide. Viscosity has a control on magma flow rates, volcano morphology, rates of gas escape, rates of convection, rates of crystal settling or flotation, rates of diffusion and crystal growth.
Viscosity of Some Common Magmas Notes : (1) rapid increase in viscosity of basaltic magma when crystals form (below ~1200ºC) and/or gas bubbles exsolve. As crystal/bubble content increases, basalts are no longer Newtonian. Flow is not initiated until a critical shear stress (yield stress: σ′ ) is exceeded. Effective viscosity: η eff = η (1-1.35 φ ) -2.5 where φ = crystal/bubble fraction. (2) Dissolved water has a strong effect on viscosity of rhyolite. H 2 O depolymerizes the silica-rich liquid by breaking network-forming Si-O-Si bonds (bridging oxygens). Obsidian (dry) 11 10 10 Pa · s at 830°C 9 E* = 122 kcal 7 Log η O H % 6 + n 2 5 a d i (Pa · s) i s b O 10 4 Pa · s at 880°C 3 E* = 56 kcal Basalt E* = 42 kcal 50 Pa · s at 1200°C 1 1156°C 977°C 838°C 727°C -1 1.0 0.6 0.7 0.8 0.9 1000 / T (°K)
a. Calculated viscosities of anhydrous silicate liquids at one atmosphere pressure, calculated by the method of Bottinga and Weill (1972) [from Hess (1989) Origin of Igneous Rocks. Harvard University Press.] b. Variation in the viscosity of basalt as it crystallizes (after Murase and McBirney, 1973), Geol. Soc. Amer. Bull., 84 , 3563- 3592. c. Variation in the viscosity of rhyolite at 1000ºC with increasing H 2 O content (after Shaw, 1965, Amer. J. Sci., 263 , 120-153). In a classic paper [Am J Sci, 272, 438 (1972)], Bottinga and Weill developed a method to calculate viscosity using the equation: ln η mix = Σ x i ln η i where x i is mole fraction of oxide i and ln η i is the viscosity contribution of component i In another classic paper [Am J. Sci, 266, 225 (1968)] Shaw et al. measured the viscosity of basaltic magma in the still molten Makaopuhi lava lake, Hawaii.
Example of application of viscosity and density data Stokes Law of settling/flotation: v = (2 r 2 g ∆ρ ) / 9 η Where v = the settling/flotation velocity r = crystal or bubble radius g = gravitational acceleration ∆ρ = density contrast between crystal or bubble and magma η = viscosity Ideally, Stokes law applies to spherical crystal and has to be modified to account for non-spherical crystals and crystal-crystal, bubble-bubble interactions. Differential settling of chromite (black) and olivine Layering in the Skaergaard Intrusion, Greenland crystals in the Stillwater Complex, Montana (maybe) showing differential crystal settling (maybe).
MOON at ~4.5 Ga Possible initial depth of lunar In a widely accepted magma ocean (~550 km) 70 km model, olivine and pyroxene settled in lunar magma ocean to 1738 km form the lunar mantle while plagioclase Primitive floated to form the Depleted Mantle lunar crust. Mantle Crust Core Ilm- Ol-Px e Plag Incr. Fe F Ol . r Px c Pl + n I Ol+Px Ol 550 km KREEP layer
Thermal conductivity of magmas Thermal conductivity (K) is a measure of the rate at which heat is conducted through rocks and magmas. [Units: J cm-1 s-1 deg-1]. Typical values for rock range from 10-2 to 2.5 x 10-2 J cm-1 s-1 deg-1. In cooling rate calculations we use the thermal diffusivity (k): k = K / ρ C (C is the specific heat). [Units of k: cm2 s-1]. To determine cooling rates, we need to solve the Fourier equation dT/dt = k [d 2 T/dx 2 ] where T is temp, t is time, x is distance Typical solution: T/T 0 = ½ + ½ erf [x / 2(kt) ½ ] where T 0 is initial temp, x = distance Thickness of crust in Alae lava lake, 0 T surface HI. Crust is stabilized at ~1065ºC (~100ºC below eruption T). Note: 10 T at t 0 linear relationship between crust t 2 thickness and square root of time Depth (ft) 20 (Peck et al., 1964) t 1 30 T country rock 40 Temperature profile in lava flow at different times 50 4 5 6 1 2 3 (months) √ t
Magmas: where are they generated and how? Source regions: locations within earth where magmas are generated, i.e., regions where the geothermal gradient intersects and exceeds the solidus at that depth. Principal source regions: Upper mantle and lower to middle crust. Partial melting: Melting requires great amount of heat (heat of fusion) so melting is always partial. At present, maximum melting in mantle is ~20%. Archean: up to 50%. Mantle: Composition: Peridotite (Ultramafic rock) composed of (in decreasing abundance) olivine, orthopyroxene, clinopyroxene and spinel (MgAl 2 O 4 ). Spinel is stable up to ~25 kb. Spinel replaced by pyrope-rich garnet (Mg 3 Al 2 Si 3 O 12 ) at higher pressures. Homogeneous on a small scale; heterogeneous on a larger scale. Only minor amounts of H 2 O (<<1%) usually contained in trace amounts of amphibole and/or biotite. Hottest mantle--beneath spreading centers. Coolest--under continental interiors. Crust: Continental crust: highly variable in thickness and composition. Lower crust: gabbro (mafic rock) or metamorphic equivalent (amphibolite). Variable H 2 O content and variable geothermal gradient. Crustal melting source regions: located above subduction zones. Geothermal gradient is higher due to advected heat in the form of basaltic magma intruded into the lower crust. H 2 O required for melting to take place. Most of this water is contained in amphiboles. Magmas are less dense than surrounding crystalline material—buoyant—therefore they will tend to rise (lava lamp). Must overcome strength of rock to form a conduit.
Partial melting of mantle peridotite 1500 (TºC) 1100 1200 1300 1400 Plag lherzolite (ol-opx-cpx-pl) Melting begins when solidus upwelling mantle 10 intersects the peridotite Spinel lherzolite 20% solidus. With decreasing (Ol-opx-cpx-sp) 50 1% pressure above the 20 20% Depth (km) solidus, extent of melting P (kbars) 10% increases. The amount of Garnet lherzolite 1% melting is limited by the (Ol-opx-cpx-gar) 30 heat available since the 100 heat of fusion is large. Extent of melting can vary 40 adiabat from ~1% to ~20%. The G r a p h T, P and % melting i t e Diamond determine the composition 50 of the basaltic magma 150 produced. 60
Recommend
More recommend