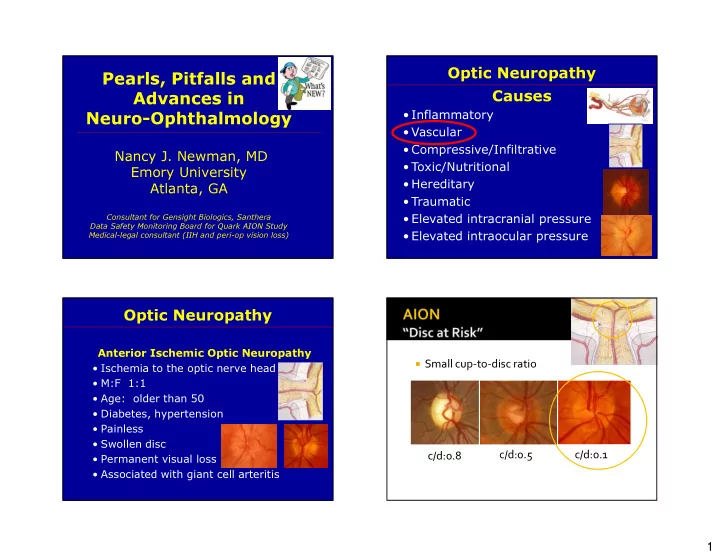

Optic Neuropathy Pearls, Pitfalls and Causes Advances in • Inflammatory Neuro-Ophthalmology • Vascular • Compressive/Infiltrative Nancy J. Newman, MD • Toxic/Nutritional Emory University • Hereditary Atlanta, GA • Traumatic Consultant for Gensight Biologics, Santhera • Elevated intracranial pressure Data Safety Monitoring Board for Quark AION Study Medical-legal consultant (IIH and peri-op vision loss) • Elevated intraocular pressure Optic Neuropathy Anterior Ischemic Optic Neuropathy � Small cup-to-disc ratio • Ischemia to the optic nerve head • M:F 1:1 • Age: older than 50 • Diabetes, hypertension • Painless • Swollen disc c/d:0.5 c/d:0.1 c/d:0.8 • Permanent visual loss • Associated with giant cell arteritis 1
AION vs ON Age Older (>50) Younger Gender M = F F > M Visual loss Acute Rapidly progressive Pain Infrequent Frequent with EOM Color Vision May be normal Commonly abnormal Distribution of age and sex in 169 young patients with AION 23% of NAION Visual Field Altitudinal defect Central defects 40 Optic Disc Acute: edema Normal or edema 35 patients are less Male ( n = 97 ) Female ( n = 72 ) Small c/d 30 than 50 years-old 25 Late: seg pallor Temporal pallor Number 20 MRI Nl optic nerve Abnl optic nerve 15 Visual prognosis Poor Good 10 5 Systemic disease HTN, DM, r/o GCA Subsequent MS 0 <25 25-29 30-34 35-39 40-44 45-49 Age at onset (years ) AION vs. ON JAMA Ophthalmol 2015; 133: 797-804 • Prognosis for visual recovery • Recognition of giant cell arteritis • Prognosis for multiple sclerosis • Treatment of demyelination 2
J Sex Med. 2015 Jan;12(1):139-51. Risk of AION increased by 3.86 when taken within the week preceding the AION vs. when taken 7 weeks prior Risk of 2.36 when taken the day before vs. within the 29 days before Giant Cell Arteritis • Rule-out giant cell arteritis (ESR, CRP, platelets) in all > 50 yo patients with ischemic optic neuropathy • Arteritic ION: • Intravitreal injection of QPI-1007 – AION or PION (small interfering ribonucleic acid – Systemic symptoms of GCA absent in 25% that blocks Caspase 2 apoptosis) • NAION 50-80 years old – Often with transient visual loss or diplopia • Within 14 days of visual loss – Bilateral if no treatment • USA – Steroids emergently, then temporal artery biopsy • India, Israel, Italy, Germany, – Poor visual prognosis Australia, and China. 3
JAMA Neurol 2015; 11: 1281 Perioperative Visual Loss Ischemic Optic Neuropathy • Anterior optic nerve ASA Registry- n=131 – Acute: swelling of disc – > 6 wks: pallor of disc Other Head and neck 10% • Posterior optic nerve 3% Cardio – Acute: normal fundus 14% pulmonary – > 6 wks: pallor of disc bypass Spine 73% • Mechanism: ischemia 4
Perioperative Visual Loss ASA Registry – Spine (n=93) CRAO Unspecified ION 11% 9% AION 20% PION 60% Anesthesiology 2012; 116: 15-24 Optic Neuropathy Toxic Optic Neuropathies Causes • Ethambutol • Inflammatory – Dose-related • Vascular – Early dyschromatopsia • Linezolide • Compressive – Dose-related • Toxic/Nutritional – Mild disc edema – Peripheral neuropathy • Hereditary • Amiodarone • Traumatic – Disc edema (mimics AION) • Cobalt-chromium metallosis • Elevated intracranial pressure – Hip implants • Elevated intraocular pressure • Methanol and ethylene glycol 5
Optic Neuropathy Causes • Inflammatory • Vascular • Compressive • Toxic/Nutritional • Hereditary • Traumatic • Elevated intracranial pressure • Elevated intraocular pressure Optic Neuropathy Optic Neuropathy Hereditary Hereditary • Maternal/Mitochondrial (Leber’s) • Autosomal dominant (Kjer’s) 6
Hereditary Optic Neuropathies Leber Hereditary Optic Neuropathy Treatment Treatment – Idebenone? • Genetic counseling -Carelli V, La Morgia C, • Symptomatic Valentino ML, et al. Idebenone treatment in • Disease-modifying Leber’s hereditary optic neuropathy. Brain • Mitochondrial diseases 2011;134:1-5/e188 • Hereditary optic neuropathies • Idebenone (900mg/d) • Gene therapy (ongoing clinical trials) Leber Hereditary Optic Neuropathy Safety and Tolerability Study Gene Therapy • Intravitreal injection of rAAV2/2- ND4 in one eye of 9 patients (3 groups escalating doses) with LHON (11778) and chronic visual loss Proc Natl Acad Sci U S A. 2012 May 15;109(20):E1238-47 • Excellent systemic safety and tolerance • No vector shedding • Good local tolerance with mild AE’s • Mild ↑IOP (23-34) and ocular inflammation: Allotopic Rescue • Treatment responsive and reversible • No unexpected adverse events NANOS, ARVO and AAO meetings, 2015 Am J Hum Genet. 2008 Sep;83(3):373-87. 7
Rescue & Reverse Studies • Two, simultaneous, parallel Phase III clinical trials of intravitreal gene therapy for the treatment of LHON, occurring at 7 study sites worldwide (Atlanta, Los Angeles, Philadelphia, Paris, London, Munich, Bologna) • Goal is to randomize 36 patients for each study over 1 year with 2-year followup From a Patient’s Perspective • Social media growth makes it easier for LHON patients and families to connect – Global LHON Facebook (2,500+ members) – Clinical database (3,400+ entries) • Website and social media facilitate study trial recruitment with just a “click” 8
Probability of Improvement vs Time Since Injury 0.7 Probability 0.6 95% CI Probability of Improvement 0.5 0.4 0.3 0.2 0.1 0.0 0 1 2 3 4 5 6 Time Since Injury (months) (2015) 9
2004 39 10
JAMA Neurol 2015; 72: 1170 11
What’s New Next Year?? – Nonmydriatic fundus photography – Idiopathic intracranial hypertension clinical trial – OCT and MS Trials – Treatment of NMO – Treatment of GCA – Ocular myasthenia gravis – LHON (Gene therapy clinical trials) – Diagnosis of concussion 12
http://novel.utah.edu/ 13
Recommend
More recommend