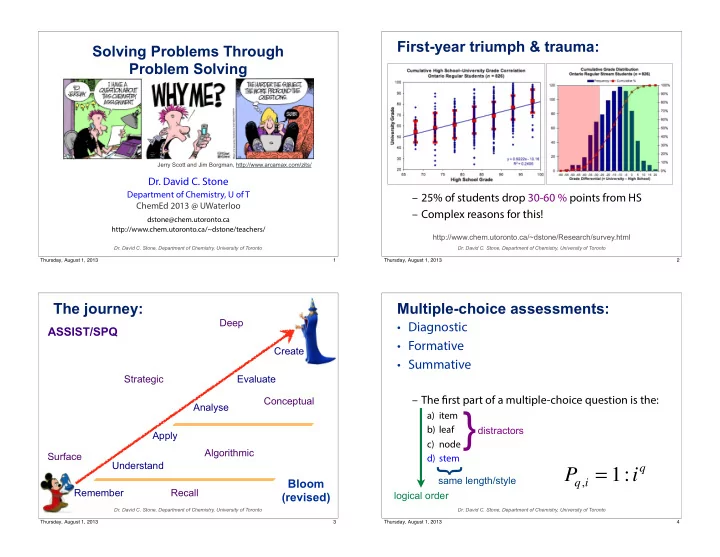

First-year triumph & trauma: Solving Problems Through Problem Solving Jerry Scott and Jim Borgman, http://www.arcamax.com/zits/ Dr. David C. Stone Department of Chemistry, U of T – 25% of students drop 30-60 % points from HS ChemEd 2013 @ UWaterloo – Complex reasons for this! dstone@chem.utoronto.ca http://www.chem.utoronto.ca/~dstone/teachers/ http://www.chem.utoronto.ca/~dstone/Research/survey.html Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 1 Thursday, August 1, 2013 2 The journey: Multiple-choice assessments: Deep • Diagnostic ASSIST/SPQ • Formative Create • Summative Strategic Evaluate – The fi rst part of a multiple-choice question is the: Conceptual Analyse d) stem } distractors a) item b) leaf Apply c) node Algorithmic Surface q , i = 1: i q Understand P } same length/style Bloom Remember Recall (revised) logical order Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 3 Thursday, August 1, 2013 4
U of T 2nd-Year Self-Test: Units and unit prefixes: • When correctly expressed in SI units, a • At a minimum, drill: density of 1.23 g/cm 3 is: – cgs ↔ mks (SI) Wrong units 2012 2011 2010 – p, n, µ, m, c, d, k, M, G a) 1.23 x 10 -3 g/m 3 17% 9% 5% – unit analysis for sanity checking Inverted b) 1.23 x 10 -3 kg/m 3 20% 19% 29% conversion c) 1.23 g/m 3 3% 4% 5% • There are six times as many students as professors at a d) 1.23 x 10 3 kg/m 3 61% 68% 62% particular university. This can be expressed mathematically as: # students equivalent to 1 professor - 6S = P # students equals multiple of # professors - S = 6P Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 5 Thursday, August 1, 2013 6 U of T 2nd-Year Self-Test: Stoichiometric calculations: • Consider the following balanced chemical reaction: • Strongly recommend the Mole Ratio! 2 MnO 4– + 16 H + + 15 I – ! 2 Mn 2+ + 5 I 3– + 8 H 2 O – law of de fi nite proportions What volume of 0.0525 M I – would be required to a A + b B ! p P + q Q MOLE RATio! exactly react with 20.0 ml of 0.0125 M MnO 4– ? 2012 2011 2010 m a n A = a ! n A = n B " inverted coefficients 8% 8% 7% a) 0.63 ml M m b 8% 5% 19% n B b b) 4.76 ml " forgot the coefficients c) 35.7 ml 77% 68% 72% d) 84.0 ml " inverted concentrations 6% 5% 2% CV PV RT Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 7 Thursday, August 1, 2013 8
U of T 2nd-Year Self-Test: U of T 2nd-Year Self-Test: • A solution of known iodine concentration may be prepared by • A 500 ml sample of a solution contains 0.375 moles of HNO 3 . mixing solutions of iodate and iodide under acidic conditions: Assuming no other acidic species are present, the pH of the 2012 2011 solution is: a IO 3– + b I – + c H + → p I 2 + q H 2 O 70% 68% a) pH = 0.125 When correctly balanced, the stoichiometric coe ffi cients in " ignored volume 18% 11% b) pH = 0.426 atoms only 2012 2011 2010 this reaction equation are: " divided by 2 (!) 3% 7% c) pH = 0.727 44% 52% 52% a) a = 1, b = 1, c = 6, p = 1, q = 3 8% 12% d) the pH cannot be less than 1 48% 41% 42% b) a = 1, b = 5, c = 6, p = 3, q = 3 charge 6% 6% 7% c) a = 3, b = 3, c = 6, p = 3, q = 3 only 2% 0% 0% d) a = 5, b = 1, c = 6, p = 1, q = 5 Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 9 Thursday, August 1, 2013 10 Write your own: Multistep calculations (algorithmic): • Form groups of ~3 • Do calculation & show your work! • Acid-base/Precipitation/Units question – m/c format with four items (a)–(d) – distractors should be common errors – share your question & critique validity Jerry Scott and Jim Borgman, November 6th 2010 http://www.arcamax.com/zits/ Handout Page 3 Handout Page 4 Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 11 Thursday, August 1, 2013 12
Multistep calculations (algorithmic): Algorithmic and conceptual m/c: • Do calculation & show your work! • Individual 0.200 g samples of each of the following gases were placed in four separate 1. write out needed equations 1.00 L stoppered flasks at 298 K. In which flask 2. combine & rearrange, etc. do you expect the gas to exert more pressure? 3. substitute values with units Explain your answer . 4. check units cancel correctly Flask: A B C D 5. calculate answer to correct s.f. 6. does fi nal value look reasonable? Gas: CH 4 CO 2 N 2 Ne • why is your answer correct? M m (g/mol) 16.0 44.0 28.0 20.2 • what assumption(s) did you make? Lillian Bird, J. Chem. Ed. , 2010 , 87(5), 541-546 Handout Page 4 Handout Page 5 Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 13 Thursday, August 1, 2013 14 Algorithmic and conceptual m/c: There are no bad questions (?) • Four fl asks of equal volume are fi lled with equal masses • Balance the following equation for the of di ff erent gases (one gas per fl ask) and sealed. If all production of ammonia: four are held at exactly the same temperature, which N 2 + H 2 → NH 3 contains gas at the greatest pressure? 2012 2011 • Represent the balanced reaction using circles 12% 25% a) Carbon dioxide (CO 2 ), M m = 44 g/mol 74% 61% with letters in the centre to depict the atoms: b) Methane (CH 4 ), M m = 16 g/mol 2% 2% c) Neon (Ne), M m = 20 g/mol d) Nitrogen (N 2 ), M m = 28 g/mol 0% 1% N N H H H H H H e) Cannot be determined 11% 10% N N H H H H H H Handout Page 10 Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 15 Thursday, August 1, 2013 16
There are no bad questions (?) There are no bad questions (?) • Two questions: • What do the following symbols indicate? – What is the pH of an acid? (a) H (b) H 2 (c) H + • Sketch a diagram to represent the metallic – What is the pH of 1.0 × 10 -8 mol/L of HCl? bonding present in a block of solid copper: • Follow-up: – Can a solution ever have a negative pH? Acid [H + ] 37% HCl 12 M 70% HNO 3 16 M 85% H 3 PO 4 15 M 96% H 2 SO 4 ~36 M Keith S. Taber, Science Education , 2003 , 87(5) , 732-758 Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 17 Thursday, August 1, 2013 18 Alternate conceptual formats: Alternate conceptual formats: • Description of experiment, phenomenon, etc. • Description of experiment, phenomenon, etc. – Mark the following explanations as either: – Mark the following explanations as either: • (T) True; (F) False; (I) Irrelevant • (T) True; (F) False; (I) Irrelevant • (E) Explains; (D) Does not explain; (I) Irrelevant • (E) Explains; (D) Does not explain; (I) Irrelevant • Many compounds of the transition metals Sc through Zinc • Roll your own, share, evaluate! have characteristic colours, both as solids and in solution. This is attributed to splitting of the 3d atomic orbitals. For example, aqueous CuSO 4 is a cyan colour because: – When an electron drops down from an upper to a lower 3d orbital, the emitted photon has a wavelength in the blue region of the spectrum – When an electron is excited from a lower to an upper 3d orbital, the absorbed photon has a wavelength in the red region of the spectrum – The increased size of the cation caused by the splitting makes it large enough Jerry Scott and Jim Borgman, http://www.arcamax.com/zits/ to scatter blue light out of solution, much like particles in the atmosphere Handout Page 11 Handout Page 12 Dr. David C. Stone, Department of Chemistry, University of Toronto Dr. David C. Stone, Department of Chemistry, University of Toronto Thursday, August 1, 2013 19 Thursday, August 1, 2013 20
Recommend
More recommend