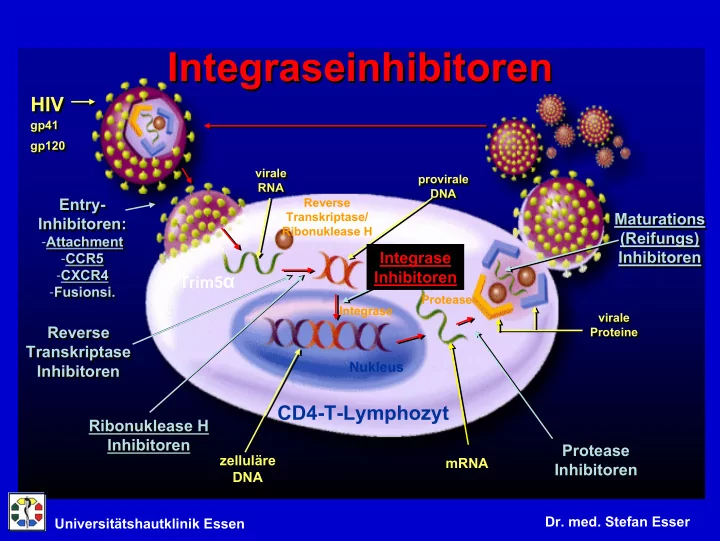

Integraseinhibitoren Integraseinhibitoren HIV HIV gp41 gp41 gp120 gp120 virale virale provirale provirale RNA RNA DNA DNA Entry- Reverse Entry- Transkriptase/ Maturations Maturations Inhibitoren: Inhibitoren: Ribonuklease H (Reifungs) (Reifungs) - Attachment - Attachment Inhibitoren Integrase - CCR5 Inhibitoren - CCR5 - CXCR4 Inhibitoren - CXCR4 Trim5 α - Fusionsi. - Fusionsi. Protease Integrase virale virale Reverse Reverse Proteine Proteine Transkriptase Transkriptase Nukleus Inhibitoren Inhibitoren CD4-T-Lymphozyt Ribonuklease H Ribonuklease H Inhibitoren Inhibitoren Protease Protease zelluläre zelluläre mRNA mRNA Inhibitoren Inhibitoren DNA DNA Dr. med. Stefan Esser Universitätshautklinik Essen
HIV-Integrase Hazuda et al 7th CROI San Fransisco 2 In-Dependent HIV-spezifische Integrase katalysiert Processing Einfügen der proviralen in Wirts-DNA Virale DNA Synthesis of 3´Ends 1 Nuclear Assembly on Viral Entry DNA in a Nuclear Membrane Nucleoprotein Complex 3a 3 Target Strand- DNA Binding Transfer Gap Repair 3b Concerted Target DNA Cleavage and Joining Mature Provirus Universitätshautklinik Essen
HIV-Integrase Hazuda et al 7th CROI San Fransisco 2 In-Dependent HIV-spezifische Integrase katalysiert Processing Einfügen der proviralen in Wirts-DNA Virale DNA Synthesis of 3´Ends HIV-1 DNA-Integrase Complex Formation Inhibitor: 1 Nuclear Assembly on Viral ~ V-165 Entry DNA in a Nuclear Membrane Nucleoprotein Complex 3a 3 Target HIV-1 Integrase Strand- DNA Transfer Inhibitors: Binding Transfer ~ S-1360 Gap Repair ~ L-708,906 3b Concerted ~ L-879810 Target DNA ~ MK-0518 Cleavage and Joining ~ GS-9137 (JTK-303) Mature Provirus Universitätshautklinik Essen
Raltegravir (MK 0518): (MK 0518): Raltegravir A Novel HIV- -1 Integrase Inhibitor 1 Integrase Inhibitor A Novel HIV A new mechanism of action: HIV-1 Integrase Transfer Inhibitor A new mechanism of action: • • Potent in vitro in vitro activity activity Potent • • ± 23 nM in 50% human serum = 33 nM ± • IC • IC 95 95 = 33 nM 23 nM in 50% human serum • Active against: Active against: • – multi multi- -drug resistant HIV drug resistant HIV- -1 1 – O K + – CCR5 and CXCR4 HIV – CCR5 and CXCR4 HIV- -1 1 O - F N N N • HIV resistant to raltegravir remain • HIV resistant to raltegravir remain H H N N sensitive to other ARTs sensitive to other ARTs O N O O • Synergistic • Synergistic in vitro in vitro with all ARTs tested with all ARTs tested Potent activity in combination therapy in Phase II studies Potent activity in combination therapy in Phase II studies • • – in ART – in ART- -naive patients naive patients at Week 24 at Week 24 (Markowitz et al, IAC 2006, Abst (Markowitz et al, IAC 2006, Abst THLB0214) THLB0214) • 85 85 – – 95% with HIV RNA < 50 copies/mL 95% with HIV RNA < 50 copies/mL • – In patients failing therapy with triple class resistant virus at In patients failing therapy with triple class resistant virus at Week Week – 24 (Grinsztejn et al, ICAAC 2006, Abst H 24 (Grinsztejn et al, ICAAC 2006, Abst H- -1670b) 1670b) • 57 57- -67% 67% with HIV RNA < 50 copies/mL with HIV RNA < 50 copies/mL • D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB Universitätshautklinik Essen
Raltegravir: BENCHMRK : BENCHMRK- -1 & 2: Study Design 1 & 2: Study Design Raltegravir • 2 identical ongoing randomized, double-blind, placebo controlled Phase III studies (in different countries) • Raltegravir 400 mg b.i.d. vs placebo (randomized 2:1) – All in combination with optimized background therapy (OBT) – Selected investigational ART permitted as OBT • Key Inclusion Criteria – Documented genotypic/phenotypic resistance to ≥ 1 drug in each of 3 classes (NNRTI + NRTI + PI) – HIV RNA > 1000 copies/mL Patients virologically failing after ≥ 16 weeks of therapy could enter an open- • label raltegravir arm (OLpVF) D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB Universitätshautklinik Essen
BENCHMRK- -1 & 2 : Baseline Patient Characteristics 1 & 2 : Baseline Patient Characteristics BENCHMRK BENCHMRK-1 BENCHMRK-2 Raltegravir + Placebo + Raltegravir Placebo + OBT OBT + OBT OBT N = 232 N = 118 N = 230 N = 119 Mean Age, yrs (SD) 46 (9) 44 (8) 45 (9) 46 (8) 46 (9) % Male 84 87 91 90 84 % Caucasian 75 81 55 65 75 Mean CD4 Count, cells/mm 3 156 153 146 163 Mean CD4 Count, cells/mm 3 156 GM Viral Load, copies/mL (log 10 HIV RNA) 40519 (4.6) 31828 (4.5) 48366 (4.7) 47789 (4.7) GM Viral Load, copies/mL (log 10 HIV RNA) 40519 (4.6) % AIDS % AIDS 94 94 90 91 92 Median Yrs of Prior ARTs (median # ART) Median Yrs of Prior ARTs (median # ART) 11 (12) 11 (12) 10 (12) 10 (12) 10 (12) % Hep B+/% Hep C+ % Hep B+/% Hep C+ 8/15 8/15 4/20 10/3 3/4 § 0/1 % GSS § 0/1 30/33 29/41 20/44 26/40 % GSS 30/33 § 0/1 % PSS § % PSS 0/1 19/29 19/29 18/33 10/34 19/27 % new enfuvirtide in OBT % new enfuvirtide in OBT 21 21 20 19 20 % new darunavir in OBT % new darunavir in OBT 27 27 25 45 50 § § GSS/PSS = total ART in OBT to which pt GSS/PSS = total ART in OBT to which pt’ ’s virus showed geno/phenotypic sensitivity by s virus showed geno/phenotypic sensitivity by Phenosense GT assay. Enfuvirtide and darunavir use in naï ïve patients were each ve patients were each Phenosense GT assay. Enfuvirtide and darunavir use in na counted as + 1 active agent and added to GSS/PSS counted as + 1 active agent and added to GSS/PSS D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB Universitätshautklinik Essen
Percent of Patients with Virologic Response Percent of Patients with Virologic Response <50 Copies/mL (Non- -Completer = Failure) Completer = Failure) <50 Copies/mL (Non BENCHMRK-1 BENCHMRK-2 HIV RNA <50 Copies/mL 100 Percent of Patients with 80 60 40 20 0 0 2 4 8 12 16 24 0 2 4 8 12 16 24 Weeks Number of Contributing Patients Raltegravir* 232 230 158 230 229 128 Placebo* 118 118 81 119 119 69 * + OBT p<0.001 at Week 16 for both parameters D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB Universitätshautklinik Essen
Partial Analysis of Raltegravir Resistance in BENCHMRK-1 and BENCHMRK-2 Partial analysis based on genotyping 41 Raltegravir failures 32 with integrase changes, 9 with no consistent changes from baseline • Virologic failure on Raltegravir vs. placebo: 76 (16%) vs. 121 (51%) • Raltegravir failure: associated with one of two genetic pathways: N155H or Q148K/R/H • Additional mutations were observed with both pathways N155H + (E92Q,V151I, T97A, G163R, L74M) Q148K/R/H + (G140S/A, E138K) • • Other pathways? Y143R/C + (L74A/I, E92Q, T97A, I203M, S230R) D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB Universitätshautklinik Essen
Combined Efficacy* (1) – – Combined Efficacy* (1) % Patients with HIV RNA < 400 copies/mL at Week 16 % Patients with HIV RNA < 400 copies/mL at Week 16 by Selected ARTs in OBT by Selected ARTs in OBT Subgroup n % of Patients 447 79 Overall Efficacy Data 230 43 Efficacy by ARTs in OBT Enfuvirtide Darunavir 44 98 + + 23 87 42 90 + - 24 63 80 90 - + 47 55 191 74 - - 90 29 0 20 40 60 80 100 + : First Use in OBT - : No Use in OBT Raltegravir + OBT Placebo + OBT * Virological failures carried forward D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB
BENCHMRK- -1 and 2 Conclusions 1 and 2 Conclusions BENCHMRK ♦ In patients with advanced HIV infection, failing ARTs with multi-drug resistant virus, raltegravir + OBT • Was generally well tolerated – with safety profile comparable to that of placebo + OBT – with few adverse experiences leading to discontinuation • Had potent and superior antiretroviral activity compared to placebo + OBT at Week 16 – Partial data at Week 24 shows similar response – When raltegravir was combined with enfuvirtide and/or darunavir, >90% achieved HIV RNA < 400 copies /mL D. Cooper, R. Steigbigel et al., 14 th CROI Los Angeles 2007, Abstract # 105 a&b LB Universitätshautklinik Essen
GS- -9137 ( 9137 (Elvitegravir Elvitegravir ): ): GS Phase 2 Study Schema Phase 2 Study Schema 278 patients • Dihydroquinoline carboxylic acid strand HIV RNA ≥ 1000 copies/mL transfer inhibitor of HIV integrase Any CD4 cell count • Boosted with 100 mg qd ritonavir ≥ 1 protease resistance mutation OBT = NRTIs +/- T-20 NNRTIs not allowed in OBT Stratified by T-20 use in OBT CPI* (n=63) GS-9137 20 mg (n=71) GS-9137 50 mg (n=71) GS-9137 125 mg (n=73) *CPI included 49% darunavir, 27% tipranavir A. Zolopa et al., 14 th CROI Los Angeles 2007, Abstract # 143 LB Universitätshautklinik Essen
Recommend
More recommend