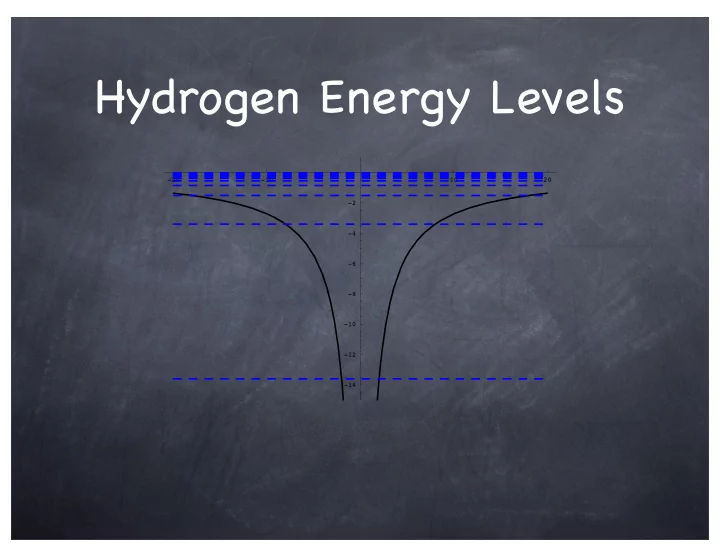

Hydrogen Energy Levels -20 -10 10 20 -2 -4 -6 -8 -10 -12 -14
hydrogen wavefunctions 5 10 15 20 -2 -4 -6 -8 -10 -12 -14
n=3 1 0.5 5 10 15 20 25 30 -0.5 -1 -1.5 -2 -2.5 -3
100
200
210
211
300
310
311
320
321
322
Lyman forest α
Angular Momentum � L = � r × � p L x = yp z − zp y , L y = zp x − xp z , L z = xp y − yp x p x → � ∂ p y → � ∂ p z → � ∂ ∂ x ∂ y ∂ z i i i [ L x , L y ] = [ yp z − zp y , zp x − xp z ] = [ yp z , zp x ] − [ yp z , xp z ] − [ zp y , zp x ] + [ zp y , xp z ] = yp x [ p z , z ] + p y x [ z, p z ] = i � ( xp y − yp x ) = i � L z [ L y , L z ] = i � L x , [ L z , L x ] = i � L y
Angular Momentum L = L 2 = L 2 L · � � x + L 2 y + L 2 z � � � � � � � � L 2 , L x L 2 L 2 L 2 = x , L x + y , L x + z , L x = L y [ L y , L x ] + [ L y , L x ] L y + L z [ L z , L x ] + [ L z , L x ] L z = L y ( − i � L z ) + ( − i � L z ) L y + L z ( i � L y ) + ( i � L y ) L z = 0 � L 2 , L y � � L 2 , L z � = 0 , = 0 � � L 2 , � = 0 L [ AB, C ] = ABC − CAB = ABC − ACB + ACB − CAB = A [ B, C ] + [ A, C ] B
Recommend
More recommend