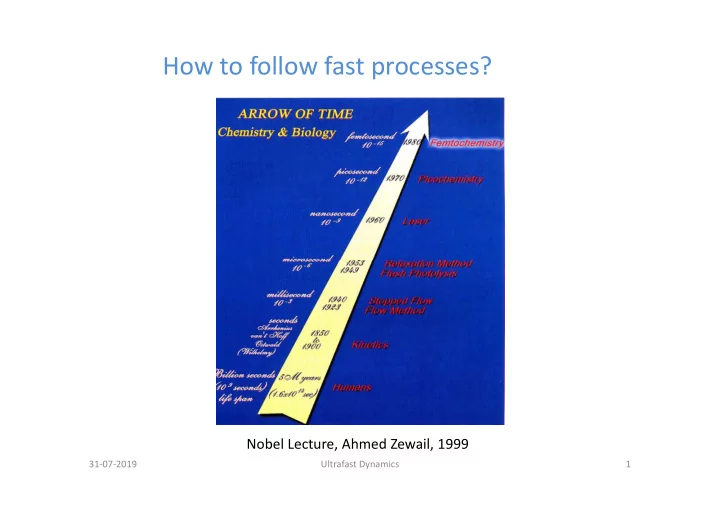

How to follow fast processes? Nobel Lecture, Ahmed Zewail, 1999 31 ‐ 07 ‐ 2019 Ultrafast Dynamics 1
Pulsed excitation Intensity Excited state population Time A = c l sample Pulsed probe light Map of time evolution Pulsed Pump light of the excited state 31 ‐ 07 ‐ 2019 Ultrafast Dynamics 2
Snapshots of bond breaking Nobel Lecture, Ahmed Zewail, 1999; Atkins, Physical Chemistry 31 ‐ 07 ‐ 2019 Ultrafast Dynamics 3
Chemistry beyond the Ground State Reactivity in the excited state is not the same as that in ground state Electronic arrangement different from that in the ground state Singlet excited state, S 1 LUMO Triplet excited state, T 1 HOMO LUMO HOMO Ground state, S o LUMO HOMO
LUMO Photoacidity HOMO Acids are more acidic in the excited state, bases are more basic Phenols: Delocalozation of electrons on O ‐ : Electron donating groups Stabilization of the phenoxide ion ‐ * transition: Delocalization involves lower energy orbitals Further stabilization of the phenoxide ion Aromatic carboxylates: Electron withdrawing groups Withdraw p electrons from the ring High electron density on O ‐ : Efficient proton abstraction ‐ * transition: * electrons are easier to withdraw More efficient proton abstraction
An example: 2 ‐ naphthol Absorption spectra Fluorescence spectra pH titration curves pH 3: protonated ground state, partially deprotonated excited state Acids are more acidic in the excited state pK a = 9.2, pK a * = 2! J. R. Lakowicz, Principles of fluorescence spectroscopy, 3 rd ed. 2006
The mechanism of acid base reactions Matteo Rini, Ben ‐ Zion Magnes, Ehud Pines, Erik T. J. Nibbering Science , 2003 , 301 , 349 – 352 Pyranine Photobase Photoacid Visible pump, Mid IR probe 31 ‐ 07 ‐ 2019 Ultrafast Dynamics 7
Dynamics from the time – resolved IR Spectra CH 3 COOH Photobase CH 3 COOH Increasing CH 3 COO ‐ concn. Smaller risetime of CH 3 COOH B. High CH 3 COO ‐ concn C. Low CH 3 COO ‐ concn
The mechanism of acid ‐ base reactions Further reading: Nibbering and coworkers, Science , 2005 , 310 , 83 ‐ 86
The take home message • There is more to Chemistry than mixing solutions • Physical Chemistry vs. Unphysical Chemistry • We need to know theory, we need to understand instruments • Look wide and never be scared of new challenges 31 ‐ 07 ‐ 2019 Ultrafast Dynamics 10
Recommend
More recommend