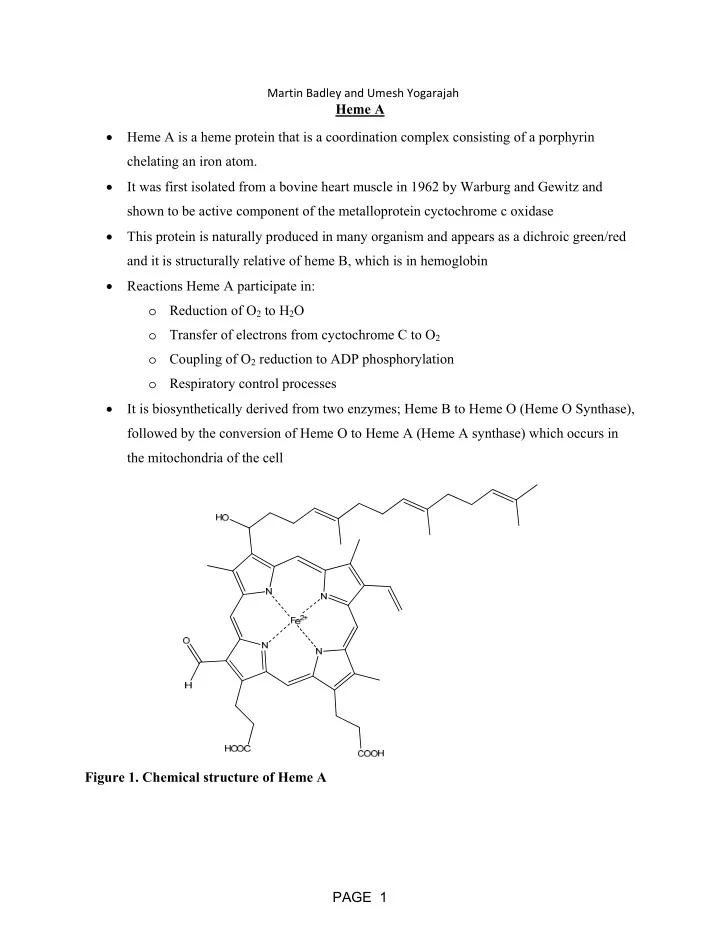

Martin Badley and Umesh Yogarajah Heme A Heme A is a heme protein that is a coordination complex consisting of a porphyrin chelating an iron atom. It was first isolated from a bovine heart muscle in 1962 by Warburg and Gewitz and shown to be active component of the metalloprotein cyctochrome c oxidase This protein is naturally produced in many organism and appears as a dichroic green/red and it is structurally relative of heme B, which is in hemoglobin Reactions Heme A participate in: o Reduction of O 2 to H 2 O o Transfer of electrons from cyctochrome C to O 2 o Coupling of O 2 reduction to ADP phosphorylation o Respiratory control processes It is biosynthetically derived from two enzymes; Heme B to Heme O (Heme O Synthase), followed by the conversion of Heme O to Heme A (Heme A synthase) which occurs in the mitochondria of the cell Figure 1. Chemical structure of Heme A PAGE 1
Jun ‐ Hyeong Park & Michael Czuczola Heme C”ysteine” The structure of Heme C is composed of a porphyrin ring with peripheral decorations Heme C is produced when the thiol groups from 2 Cysteines form covalent thioether bonds with the vinyl groups of Heme B o Heme B is attached to a CysXXCysHis pentapeptide segment, where His serves as an axial ligand to the Fe, to form Heme C in Cytochrome C Heme C is vital to the electron transport chain as it functions as an electron carrier in Cytochrome C (proteins that contain Heme C are refer to as cytochromes c) Cytochrome C can initiate cell apoptosis by releasing from the mitochondria, which will trigger a series of biochemical reactions to activate cell death Heme C has a large range of reduction potentials in nature that spans over 1V. Cytochrome C cycles between the reduced ferrous and oxidized ferric states to transfer a single electron o Useful in determining the thermodynamics of electron transfer reactions o Useful in observing electron transfer kinetics Heme C has to be used instead of Heme B in Cytochrome C because Heme C binds more tightly with covalent bonds while Heme B will dissociate out of the protein because it does not have a high enough binding affinity to the amino acid segment of the motif Heme C is the only heme that strictly only participates in electrochemistry. Both of the axial positions of the iron centre are blocked off by His and Cys 2 x Cysteine Heme B Heme C PAGE 2
4-BACTERIOCHLOROPHYLL-A By: Claire Tully and Keenan Fast Bacteriochlorophyll-a is found in purple bacteria, a anoxygenic phototroph, and absorbs wave lengths of 805nm, and 830-890nm. Bacteriochlorophyll-a is composed a porphyrin ring that the has two reduced pyrrole groups which is called a bacteriochlorin ring. This type of chlorophyll is found in the simplest life forms, that evolved about 3.5 billion years ago. During their evolution, the light assessable was lacking in blue and red light due to dust and other debris in the atmosphere. These bacteria could harness low energy light to sustain NADP production and survive. As the debris cleared, the blue light could access organisms which resulted in the evolution of chlorophyll, and thus able to absorb higher energy wave lengths. Within the purple sulfur bacteria, photosynthesis occurs using sulfur as a source of electrons. Sulfur was used for bacteria rather than oxygen because the bacteria are absorbing lower energy wavelengths in comparison to plants. The lower light energy wave lengths provided enough energy to remove the electron from sulfur however was not enough energy to remove an electron from oxygen. The lamellae in the bacteria have several membrane proteins in which house light activation centers. The light harvesting complex II is composed of alpha and beta proteins that form a ring like structure surrounding light harvesting complex I(LHI). LH1 is composed of bacteriochlorophyll-a as well as carotenoids that are collectively the reaction center. From this complex photosynthesis can occur. PAGE 3
Heme o Heme o[Mg] Brianne Potts and Maissa Belcina Heme o was first discovered in cytochrome bo 3 -type QOX in 1991. Cytochrome bo 3 -type QOX is a terminal oxidase in the aerobic respiratory chain of E. coli. Heme o is contained in Subunit I and forms a binuclear center with Cu B where dioxygen is reduced to water. It is also used for aerobic respiration in archaebacteria, bacteria and eukaryotes. Heme o is synthesized from heme b through selective farnesylation in the presence of farnesyl diphosphate (FPP), divalent cations (Mg 2+ or Ca 2+ ), and reducing agents like dithionite. It also acts as the precursor for heme A. The two heme groups differ at ring position 8 where heme o has a methyl substituent, whereas heme A contains a formyl group. Tatsushi, M. The Porphyrin Handbook; Kadish, K.M.; Smith, K.M.; Guilard, R.; Academic Press: San Diego, 2003; Vol. 12; p 158. PAGE 4
5-Heme m By: Chris Aspros Heme m also known as methemoglobin and is a heme found in the blood that is very close to heme B. The only difference is that the iron is in the ferric state rather than the ferrous state. Since the iron is in a higher oxidation state methemoglobin does not bind to oxygen and increases the affinity of oxygen for other oxygen binding hemoglobin. It is normal to have about 1-2% of heme in the blood as methemoglobin. Heme m is reduced into heme B via two pathways. The first is the more dominant path and it involves NADH and cytochrome b5 and cytochrome b5 reductase. The second uses NADPH and methemoglobin reductase and this can become the main pathway if methylene blue is added as a cofactor. Once reduced the heme group can accept oxygen again. One condition characterized by having too much methemoglobin in ones system is called methemoglobinemia and can be both genetic and acquired. Some conditions include cyanosis of the skin and as the amount of methemoglobin concentration in the blood increases conditions can worsen to include dizziness headaches and death. The main treatment for acquired methemoglobinemia is to take in methylene blue PAGE 5
Heme ‐ B (not in Mb or Hb) By Drishti Kataria and Matthew Baistrocchi - The most abundant Heme How does it work in… COX ‐ 2 COX ‐ 2 (cyclooxygenase) has 2 functions: 1. the oxidation of arachidonic acid to ProstaglandinG2 (PGG2) 2. reduction of PGG2 to form ProstaglandinH2 (PGH2) ‐ 2 active sites: heme group and cyclooxygenase site ‐ Heme, a prosthetic group, is involved in both the peroxidase and cyclooxygenase reactions. ‐ In the cyclooxygenase active site: heme binds to His ‐ 388 and interacts with Tyr ‐ 385 forming a tyrosine radical which is necessary for the formation of PGG2 from arachidonic acid ‐ In the peroxidase active site: heme acts as the reducing agent in the reduction of PGG2 to form PGH2 Peroxidase a large group of oxidoreductases that catalyze the oxidation of substrate molecules using hydrogen peroxide as electron acceptor ‐ Contain heme as cofactor ‐ A 2 electron reduction where H2O2 is reduced to H2O and the enzyme is oxidized PAGE 6
Cobalamin aka Vitamin B12 Devon Chapple and Kyle Jackman ‐ 1 of 8 B vitamins; 4 major chemical forms of vitamin B 12 : Cyanocobalamin, hydroxocobalamin and the two coenzyme forms of vitamin B12 (methylcobalamin and adenosylcobalamin) ‐ Cyanocobalamin o Treat pernicious anemia and can be converted to any active vitamin B 12 compound ‐ Hydroxocobalamin o Treat pernicious anemia, cyanide poisoning, optic atrophy, and toxic amblyopia ‐ Methylcobalamin o Treatment of peripheral neuropathy, diabetic neuropathy, and preliminary treatment of amyotrophic lateral sclerosis ‐ Adenosylcobalamin o Cofactor to the methylmalonyl ‐ CoA mutase enzyme ‐ Overall functions of vitamin B 12 compounds: red blood cell formation, functioning of the brain and the nervous system, as well as the synthesis and regulation of DNA. Used in the metabolism of every cell in the body, and helps to facilitate the release of energy. ‐ Cobalt metal center in either the +1 or +2 oxidation state. ‐ Only eukaryotic cells are able to naturally synthesize cobalamin and as a result all of the cobalamin in our bodies must be ingested. ‐ Vitamin B 12 deficiency can lead to: pernicious anemia, irritability, low red blood cell count, and decreased fertility H 2 NOC CONH 2 R H 2 NOC N N CONH 2 Co + N N H 2 NOC CONH 2 N O N HN HO O O OH O P O - O ‐ PAGE 7
Recommend
More recommend