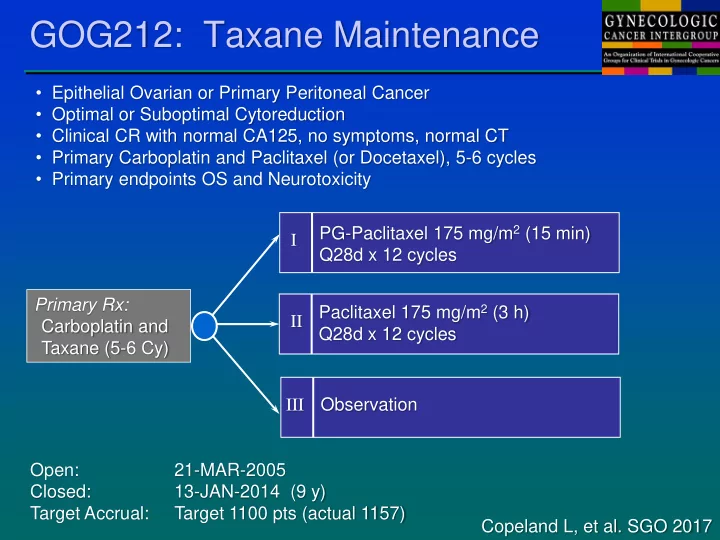

GOG212: Taxane Maintenance • Epithelial Ovarian or Primary Peritoneal Cancer • Optimal or Suboptimal Cytoreduction • Clinical CR with normal CA125, no symptoms, normal CT • Primary Carboplatin and Paclitaxel (or Docetaxel), 5-6 cycles • Primary endpoints OS and Neurotoxicity PG-Paclitaxel 175 mg/m 2 (15 min) I Q28d x 12 cycles Primary Rx: Paclitaxel 175 mg/m 2 (3 h) II Carboplatin and Q28d x 12 cycles Taxane (5-6 Cy) Observation III Open: 21-MAR-2005 Closed: 13-JAN-2014 (9 y) Target Accrual: Target 1100 pts (actual 1157) Copeland L, et al. SGO 2017
GOG212: Taxane Maintenance HR (97.5% CI) HR (97.5% CI) CT-2103 vs OBS 0.847 (0.721 - 0.995) CT-2103 vs OBS 0.979 (0.781 - 1.23) Paclitaxel vs OBS 0.783 (0.783 - 0.921) Paclitaxel vs OBS 1.104 (0.884 - 1.38) No established role for maintenance therapy using conventional cytotoxic agents, based on multiple phase III trials Copeland L, et al. SGO 2017
NRG-GY007: NACT +/- Ruxolitinib • Epithelial ovarian, peritoneal, or fallopian carcinoma (EOPFC) • Stage IIIC-IV and suitable for NACT with interval cytoreductive surgery • Phase I to evaluate acute toxicity (C1) and cumulative tolerability • Maintenance ruxolitinib permitted in patients tolerating concurrent therapy • Primary Endpoints: PFS and molecular targeting (stem cells and IL6) CP (x3) ICS CP (x3) Observation 1:2 Core Bx R CP (x3) CP (x3) Rux Maint ICS + Rux + Rux (optional) CP = Carboplatin AUC 5 or 6 (D1), Paclitaxel 80 mg/m2 (D1,8,15) Rux = Ruxolitinib 10-15 mg PO BID (pending Phase 1) ICS = Interval Cytoreductive Surgery Open: 10-OCT-2016 Closed: (ongoing phase I) Accrual: Burger R, for NRG Oncology
GOG-3015: Chemo Bev ± Atezo YO39523/GOG-3015/ENGOT-ov39 • Previously untreated high-grade cancer • Stage III macroscopic or Stage IV (allows election of NACT), Bx cohort • No history of serious autoimmune disorders • Stratification PDL1 0 vs 1+, Stage, PS, NACT • Co-Primary endpoints (PDL1+): OS HR 0.72 (81%, 0.046), PFS HR 0.7 Carboplatin AUC=6 D1 Bevacizumab 15 mg/kg Paclitaxel 175 mg/m 2 D1 I Placebo Bevacizumab 15 mg/kg D1 (q3w x 16 cycles) Placebo IV D1 R Carboplatin AUC=6 D1 Bevacizumab 15 mg/kg D1 Paclitaxel 175 mg/m 2 D1 II Atezolizumab 800 mg D1 Bevacizumab 15 mg/kg D1 (q3w x 16 cycles) Atezolizumab 800 mg D1 Open: MAR 2017 Closed: (ongoing) Target Accrual: 1300 Moore K and Pignata S, for NRG-F and ENGOT
NRG-GY009: PLD ± Atezo ± Bev • Recurrent high-grade with PFI < 6 months (following most recent platinum) • No more than 2 prior regimens (including primary therapy) • RECIST measurable or evaluable disease with accessible tumor • No prior anti-angiogenic therapy for platinum-resistant recurrence • No history of serious autoimmune disorders • Primary endpoints: Phase II PFS (selective) Phase III OS PLD 40 mg/m 2 IV q4w HR PFS ≤ 0.783 (88% power) I Atezolizumab 800 mg IV q2w HR OS* ≤ 0.625 (90% power) *one-tail α 0.0115 PLD 40 mg/m 2 IV q4w (multiple comparisons) R II Bevacizumab 10 mg/kg IV q2w Atezolizumab 800 mg IV q2w PLD 40 mg/m 2 IV q4w III Bevacizumab 10 mg/kg IV q2w Open: 12 MAY 2017 Closed: (ongoing safety lead-in, Arm I, non-randomized) Target Accrual: 272 Phase II, Cumulative 488 Phase III O'Cearbhaill RE, for NRG
PARP Inhibition… Who? What? Where? When? Why? CSI: C hemo S cene I nvestigation
GOG3005: PARPi Primary Therapy • High-grade extrauterine serous tumors, Stage I-C, II, III, IV • Election for NACT-ICS and scheduling of paclitaxel (no IP therapy) • Primary endpoint PFS: (1) Entire Population, (2) BRCA1/2 Population • Stratifications: Stage, Residual Disease, NACT-ICS, Region, gBRCA status Paclitaxel (standard or dose-dense) Placebo Carboplatin AUC 6 (IV)* x 6 I PO BID Placebo PO BID 1:1:1 Paclitaxel (standard or dose-dense) Placebo Carboplatin AUC 6 (IV)* II x 6 PO BID Veliparib 150 mg PO BID Paclitaxel (standard or dose-dense) Veliparib 400 mg Carboplatin AUC 6 (IV)* II x 6 PO BID Veliparib 150 mg PO BID Collaborative development with AbbVie (M13-694) including international participation, seeking EMA and FDA regulatory approval Open: JUL 2015 Closed: MAY 2017 Target Accrual: ~1100 pts (264 BRCA1/2 +) Coleman R, for GOG Foundation
NRG-GY004: PARPi +/- Cediranib • Recurrent HGSC with PFI > 6 months (following most recent platinum) • No more than 3 prior regimens (including primary therapy) • RECIST measurable or evaluable disease with accessible tumor • No prior PARPi therapy, prior bevacizumab permitted • Stratify for BRCA status, number of prior treatment regimens • Primary endpoint: PFS 85% Power with HR 0.625 Olaparib 300 mg BID Projected interim analysis JUL 2018 Cediranib 30 mg QD R Olaparib 200 mg BID Platinum-based combo* (IV) *Carboplatin + gemcitabine or paclitaxel or PLD Open: FEB 2016 Closed: (ongoing) Target Accrual: 550 pts (135 BRCA1/2 +) Liu J, for NRG
NRG-GY005: PARPi +/- Cediranib • Recurrent HGSC with PFI < 6 months (following most recent platinum) • No more than 2 prior regimens (including primary therapy) • RECIST measurable or evaluable disease, biopsy accessible • No prior PARPi therapy, prior bevacizumab permitted • Stratify for BRCA status, number of prior treatment regimens • Primary endpoint: OS 90% Power with HR 0.625 Phase II (n = 180) Phase III (n = 280) Cediranib (PO) Selected Regimen (PO) Olaparib (PO) 1:1 R R Non-Platinum Chemo* (IV) Cediranib + Olaparib (PO) * Weekly paclitaxel or PLD Non-Platinum Chemo* (IV) Open: FEB 2106 Closed: (ongoing) Target Accrual: 460 pts (135 BRCA1/2 +) Lee J-M, for NRG
PARPi: Maintenance vs Treatment What are the endpoints?
PARPi: Maintenance vs Treatment • Emerging data with maintenance and treatment is compelling, with an immediate impact on regulatory approvals and PARPi utilization • Should control of small-volume asymptomatic disease be our goal? Timing and sequence has not been addressed in any prospective clinical trial… • It is difficult to monitor ongoing response in a maintenance setting with normal CA125 and imaging (+/- secondary cytoreduction), and many patients could receive long-term ineffective therapy • Consider the importance of balancing treatment-related toxicity, risk of symptomatic recurrence, and time off-therapy (in a non-curative setting) • Current long-term PARPi treatment is associated with emergence of resistance, potentially limiting subsequent therapeutic benefit
“Generally Well - Tolerated” • 78 year-old, recurrent HGSC, Stage III-C, originally diagnosed 2001 • gBRCAwt, multiple lines of therapy, new pulmonary nodules in 2015 • Living independently, asymptomatic • Enrolled on a Phase II trial with PARPi • Near-complete response, all lesions • Dose-limiting toxicity requiring multiple dose reductions and treatment interruptions, including anemia (Hgb 7.6), Plts, weakness, fatigue, nausea, appetite, weight • Treatment self-discontinued after one year Pre-Rx NOV2015 Post-Rx DEC2016 • PARPi symptoms resolved to baseline within 4 weeks • Minor progression on CT imaging at 5 months • No additional therapy... Bookman MA 2017
Therapance : PARPi Therapy vs Maintenance • Enroll patients at completion of primary therapy (CR or PR) • Flexible allowance for chemotherapy, PARPi, and utilization of bevacizumab, (reflecting local standards and regulatory approvals) • Minimized data collection to limit study cost • Primary Endpoint: OS at 3 Years (from diagnosis) • Secondary Endpoints: Cumulative time on/off therapy, etc. Chemo +/- Bev 2L Treatment PARPi CR, PR Recurrence Maintenance Primary Therapy PARPi PARPi R +/- Bevacizumab 2L Treatment Maint OBS +/- Bev R R Maintenance Chemo +/- Bev OBS 2L Treatment ?? Bookman MA (for anyone interested)
Recommend
More recommend