
Enzymes! Biology
Intro Video • https://www.youtube.com/watch?v=XTUm- 75-PL4
• An enzyme is a protein that functions as a catalyst to speed up a chemical reaction in the body. • Continuously recycled over and over again. • Biological catalyst: speed up rates of reactions inside the cytoplasm. • They control the rate of metabolic reactions in the body. • They lower activation energy (energy needed to get a reaction started). • They weaken chemical bonds so molecules can be made or broken down by the body.
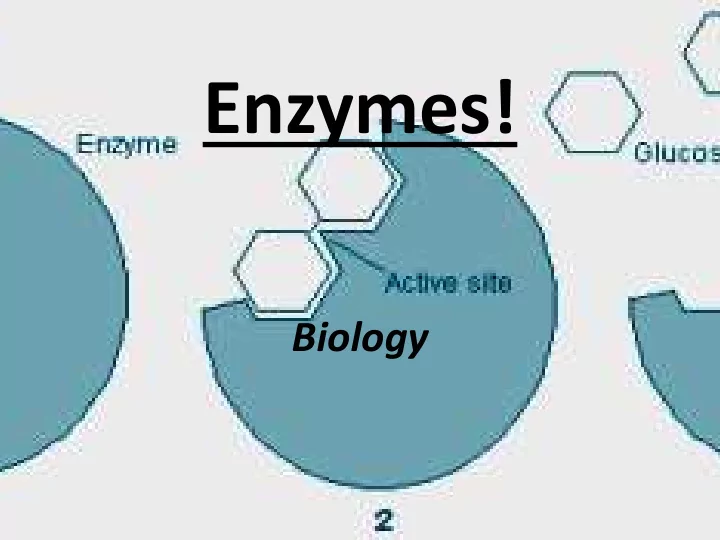
• Highly specific: catalyze only one chemical reaction at a time and they have a specific substrate. Specific Terminology • Substrate : the reactant in the chemical reaction that is catalyzed by the enzyme, the substance that is changed. • Active Site: the region on the enzyme where the substrate attaches. The shape of the active site changes based on the substrate. • Product: what is created after the chemical reaction has occurred.
• Many enzymes have an “ ase ” ending . • A few popular ones: – Catalase-> breaks down hydrogen peroxide in eukaryotic cells – Sucrose (table sugar) -> sucrase – Lipids-> lipase – Proteins-> Proteases – Amylase-> human saliva, helps break down starch (amylose) – Lactose -> Lactase – DNA helicase-> unzips the double stranded DNA molecule for replication – DNA polymerase-> enzymes that create DNA molecules by assembling nucleotides
Fun Fact! • Catalase breaks down hydrogen peroxide in eukaryotic cells. • It’s estimated that 40 million hydrogen peroxides go into a catalase enzyme every second. • Hydrogen peroxide is toxic to our cells in large quantities https://www.youtube.com/watch?v=3Tn- 7JcZJuQ&ebc=ANyPxKroxLtgDhGJngKVMtc5s- x0rTynj- TXwGMvf4hSY5G_i7nJkJc9ampYnrF6krm- 1RzRCN8DzYTBNcZTRYSUG_AquKdc8g&spfrelo ad=10
Enzyme Substrate Complex • Steps: – 1. Substrate binds to the enzyme at the active site. – 2. The substrate is changed by the enzyme and converts the reactants into products. – 3. Products are released into the body. – 4. Once the products are released, the active site is ready for another molecule (substrate) to bind to. – 5. The process is ongoing, it never stops.
• This is often referred to as the “Lock and Key Model” or the “Enzyme Substrate Complex” • The shape of the active site (“the lock”) determines which substrate (“the key”) will “fit” into the enzyme.
• Lowers activation energy so that the product can form and the chemicals can spontaneously break apart. • Picture from Biology Holt McDougal text, 2012
Factors that Affect Enzymes • 1. Temperature: • As temperature increases, kinetic energy increases, and molecules move around a lot more. The more they move around, the higher the probability that an enzyme and a substrate will bind together and react. – Enzymatic reactions increase with an increase in temperature.
• Works best at a certain pH. – Sensitive to changes in pH, especially acidity – Too low or too high, the enzyme will DENATURE (fall apart).
https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=images&cd=&cad=rja&uact=8&ve d=0ahUKEwjv66SYoJ7LAhXMGR4KHez1CdMQjB0IBg&url=http%3A%2F%2Fwww.rsc.org%2Flearn -chemistry%2Fresources%2Fchemistry-in-your- cupboard%2Fvanish%2F8&psig=AFQjCNFN6wFjYVL_Vc6UFl- cb2Bf7Iu5Ag&ust=1456875515003082
3. Concentration of Enzyme or Substrate If enzyme concentration is low, the reaction is • As the enzyme slow. concentration increases, the rate of reaction increases. http://www.rsc.org/Education/Teachers/Resou rces/cfb/enzymes.htm
• As the substrate increases, the reaction increases, up to a certain point (enzyme is limited). http://faculty.clintoncc.suny.edu/faculty/micha el.gregory/files/bio%20101/bio%20101%20lab oratory/enzymes/enzymes.htm
Activity / Homework • Complete the Enzyme- Substrate Manipulation Activity
Enzyme Substrate Complex: Graphing and Manipulative Lab
Videos • https://www.youtube.com/watch?v=NdMVRL 4oaUo • https://vimeo.com/86362472
ATP & Energy Our main energy currency
• ATP= Adenosine Triphosphate • Macromolecule: Nucleic Acid • Organelle: Mitochondria • Main Function: Main energy currency in all living things. • Comes from: breakdown of glucose (carbohydrates).
Structure: Made up of adenine, ribose, and three phosphates
Differences ATP ADP 3 phosphates 2 phosphates High energy Low energy
phosphate removed
• Fats store the most energy. – 80 % of the energy in your body. – About 146 ATP molecules from a triglyceride. • Proteins are the least likely to be broken down to make ATP because they have so many different functions. – Amino acids are not usually needed for energy. – About the same amount of energy as a carbohydrate. • Carbohydrates are the molecules most commonly broken down to make ATP. – Not stored in large amounts. – Up to 36 ATP is created from one glucose molecule.
Of course it all starts with photosynthesis, and then organisms take it in via cellular respiration • What if there isn’t any sunlight? Where does the energy come from? – Chemosynthesis: process by which organisms use chemical energy to make their food. – Where does this occur? • Deep sea hydrothermal vents.
https://www.youtube.com/watch?v=XotF9fzo4 Vo
Review Questions • 1. Explain in your own words, what is occurring in the ATP / ADP cycle. • 2. Describe two functions of catalysts in chemical reactions. • 3. The substrate is also known as the _________________ in a chemical reaction. • 4. List three ways in which enzymes can be altered. • 5. Some organisms live in very hot or very acidic environments. Would their enzymes function in a person’s cells? Why or why not? • 6. Suppose that the amino acids that make up an enzyme’s active site are changed, how might this change affect the enzyme? • 7. What is the main function of ATP? • 8. How do we obtain ATP? • 9. Which organic molecule is used by the body as a good source of long term energy storage? • 10. Proteins are composed of chains of ______________________
Label the Diagram
Recommend
More recommend