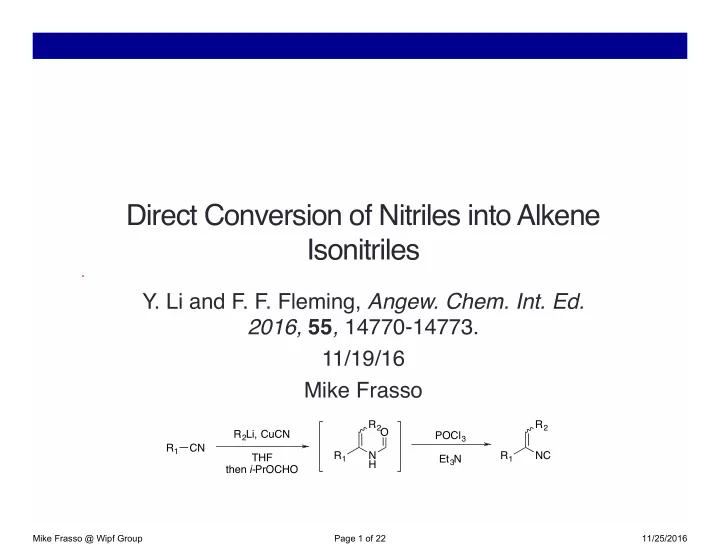

Direct Conversion of Nitriles into Alkene Isonitriles � Y. Li and F. F. Fleming, Angew. Chem. Int. Ed. 2016, 55 , 14770-14773. � 11/19/16 � Mike Frasso � R 2 R 2 O R 2 Li, CuCN POCl 3 R 1 CN R 1 N R 1 NC THF Et 3 N H then i- PrOCHO Mike Frasso @ Wipf Group Page 1 of 22 11/25/2016
Most Prominent Physical Characteristic…Stench � • ‘highly specific, almost overpowering’, ‘horrible’, and ‘extremely distressing’ • To quote odor theorist Luca Turin, “isonitriles” are just the Godzilla of smells, you can’t believe how awful they smell, they make you vomit your guts out instantly.” • Have been used in nonlethal weaponry • “The containment vessel may be comprised of a "paint ball", grenade, non-lethal land mine, spray bottle, rubber bullet, a modified shotgun shell, or other like vessel.” Burr, C. The Emperor of Scent: A Story of Perfume, Obsession, and the Last Mystery of the Senses ; Random House: New York, 2002. US Pat. 6 352 032, March 5, 2002 J. Org. Chem. 2009 , 74 , 4110–4117 Mike Frasso @ Wipf Group Page 2 of 22 11/25/2016
Two General Reaction Pathways � Nu - E + R N E R N R N Nu E + Nu - Nu Nu N N R E R E Isonitrile Nucleophilicity NC O NC NC ~ > ~ > ~ OTMS NC TMS Angew. Chem. Int. Ed. 2007 , 46 , 3563-3566. � Mike Frasso @ Wipf Group Page 3 of 22 11/25/2016
Some Characteristic Reactions of Isonitriles � • Heterocycle synthesis: furans, pyrroles, oxazoles, etc. Ts NC N O K 2 CO 3 van Luesen Reaction O Ph MeOH, reflux Ph • Ugi NC R 3 O O R 2 R 1 R 1 N R 4 N H R 4 COOH R 3 O NH 2 R 2 • Passerini R 3 O O NC R 1 R 1 O R 4 N H apolar solvent R 3 O R 4 COOH Mike Frasso @ Wipf Group Page 4 of 22 11/25/2016
Preparation of Isonitriles � • Most Common Methods: • Substitution: X = halide, activated ester, O-PR 3 ; various CN sources possible AgCN X R 1 R 1 NC R 2 R 3 R 2 R 3 • Dehydration O POCl 3 , Et 3 N HN R 1 CN R 1 R 2 R 3 R 2 R 3 Mike Frasso @ Wipf Group Page 5 of 22 11/25/2016
Preparation of Alkene Isonitriles � � Tetrahedron Lett . 1989 , 30 , 3335-3338. � . � 1. AgOCN, I 2 , Et 2 O 2. Cl 3 SiH, Et 3 N, CH 2 Cl 2 , 0 o C 3.KO t Bu, THF O Synlett 1990 , � � Tf 2 O, DIPEA Cu 2 O 603-604. � Tetrahedron 1971 , 27 , N NC NC 3795-3801. CH 2 Cl 2 H . 1. KHMDS, -78 o C, THF 2. O (EtO) 2 P NC J. Org. Chem. 2009 , Ph 3 P NC 74 , 4110-4117. � Cl- O NCSe NC P(OR) 3 [3,3] � SeCN Angew. Chem. Int. Ed. 1995 , 34 , 1627-1629 . � Mike Frasso @ Wipf Group Page 6 of 22 11/25/2016
Preparation of Alkene Isonitriles � � Tetrahedron Lett . 1989 , 30 , 3335-3338. � . � 1. AgOCN, I 2 , Et 2 O 2. Cl 3 SiH, Et 3 N, CH 2 Cl 2 , 0 o C 3.KO t Bu, THF O Synlett 1990 , � � Tf 2 O, DIPEA Cu 2 O 603-604. � Tetrahedron 1971 , 27 , N NC NC 3795-3801. CH 2 Cl 2 H . 1. KHMDS, -78 o C, THF 2. O (EtO) 2 P NC J. Org. Chem. 2009 , Ph 3 P NC 74 , 4110-4117. � Cl- O NCSe NC P(OR) 3 [3,3] � SeCN Angew. Chem. Int. Ed. 1995 , 34 , 1627-1629 . � Mike Frasso @ Wipf Group Page 7 of 22 11/25/2016
Preparation of Alkene Isonitriles � � Tetrahedron Lett . 1989 , 30 , 3335-3338. � . � 1. AgOCN, I 2 , Et 2 O 2. Cl 3 SiH, Et 3 N, CH 2 Cl 2 , 0 o C 3.KO t Bu, THF O Synlett 1990 , � � Tf 2 O, DIPEA Cu 2 O 603-604. � Tetrahedron 1971 , 27 , N NC NC 3795-3801. CH 2 Cl 2 H . 1. KHMDS, -78 o C, THF 2. O (EtO) 2 P NC J. Org. Chem. 2009 , Ph 3 P NC 74 , 4110-4117. � Cl- O NCSe NC P(OR) 3 [3,3] � SeCN Angew. Chem. Int. Ed. 1995 , 34 , 1627-1629 . � Mike Frasso @ Wipf Group Page 8 of 22 11/25/2016
Preparation of Alkene Isonitriles � � Tetrahedron Lett . 1989 , 30 , 3335-3338. � . � 1. AgOCN, I 2 , Et 2 O 2. Cl 3 SiH, Et 3 N, CH 2 Cl 2 , 0 o C 3.KO t Bu, THF O Synlett 1990 , � � Tf 2 O, DIPEA Cu 2 O 603-604. � Tetrahedron 1971 , 27 , N NC NC 3795-3801. CH 2 Cl 2 H . 1. KHMDS, -78 o C, THF 2. O (EtO) 2 P NC Org. Lett. 2016 , 18 , Ph 3 P NC 1622-1625. � Cl- O NCSe NC P(OR) 3 [3,3] � SeCN Angew. Chem. Int. Ed. 1995 , 34 , 1627-1629 . � Mike Frasso @ Wipf Group Page 9 of 22 11/25/2016
Preparation of Alkene Isonitriles � � Tetrahedron Lett . 1989 , 30 , 3335-3338. � . � 1. AgOCN, I 2 , Et 2 O 2. Cl 3 SiH, Et 3 N, CH 2 Cl 2 , 0 o C 3.KO t Bu, THF O Synlett 1990 , � � Tf 2 O, DIPEA Cu 2 O 603-604. � Tetrahedron 1971 , 27 , N NC NC 3795-3801. CH 2 Cl 2 H . 1. KHMDS, -78 o C, THF 2. O (EtO) 2 P NC Org. Lett. 2016 , 18 , Ph 3 P NC 1622-1625. � Cl- O NCSe NC P(OR) 3 [3,3] � SeCN Angew. Chem. Int. Ed. 1995 , 34 , 1627-1629 . � Mike Frasso @ Wipf Group Page 10 of 22 11/25/2016
Isonitriles from Benzoxazoles � N NC 1. BuLi, THF 2. RCOCl O O R O NC NC O O O O 85% 92% "malt" "natural rubber" NC NC O O O O O CN 83% 90% "taffy" "old wood" � J. Org. Chem. 2009 , 74 , 4110–4117 . Mike Frasso @ Wipf Group Page 11 of 22 11/25/2016
“Convertible” Isonitriles � O R 2 N R 3 O R 2 O HCl N R 3 R 1 O N H MeOH + O O R 1 O N R 4 R 4 O • Definition: Resulting amide from Ugi reaction can be readily activated for further transformations MeO 2 C CO 2 Me PMB O PMB Ph N HCl N N H O O Ph O PhMe, 100 o C MeO 2 C CO 2 Me 59% p -Tol PMB Ph N O O � J. Org. Chem. 2009 , 74 , 4110–4117 . Mike Frasso @ Wipf Group Page 12 of 22 11/25/2016
“Convertible” Isonitriles � O R 2 N R 3 O R 2 O HCl N R 3 R 1 O N H MeOH + O O R 1 O N R 4 R 4 O MeO 2 C CO 2 Me PMB O PMB Ph N HCl N N H O O Ph O PhMe, 100 o C MeO 2 C CO 2 Me 59% p -Tol PMB Ph N O O � J. Org. Chem. 2009 , 74 , 4110–4117 . Mike Frasso @ Wipf Group Page 13 of 22 11/25/2016
A Few Possibilities… � H 2 O O O MeLi H 2 O X R 2 Ph CN Ph NLi Ph N R 2 Ph O +/- H + +/- H + R 2 O O O O O O X R 2 X R 2 X R 2 Ph NH 2 Ph NHLi Ph N R 2 Ph N R 2 H R 2 O � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 14 of 22 11/25/2016
A Few Possibilities… � H 2 O O O MeLi H 2 O X R 2 Ph CN Ph NLi Ph N R 2 Ph O +/- H + +/- H + R 2 O O O O O O X R 2 X R 2 X R 2 Ph NH 2 Ph NHLi Ph N R 2 Ph N R 2 H R 2 O � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 15 of 22 11/25/2016
Foundation of this Work � O MeLi,LiBr R CN R N Et 2 O H then Ac 2 O F O O S N N H H F 85% 60% O O N N H H O Cl 62% 90% � Org. Lett. 2006 , 8, 3903-3906 . � � Mike Frasso @ Wipf Group Page 16 of 22 11/25/2016
Screen of Conditions � O MeLi, MX POCl 3 Ph CN THF Ph N Ph NC Et 3 N H then i- PrOCHO Entry Metal Salt (mol%) Yield (%) 1 ---- 39 • Using methyl formate favored formyl imine 2 LiBr (120) 30 • Grignard reagents gave 3 CuCN (10) 43 complex mixtures 4 CuI (10) 40 N CuBr . SMe 2 (10) 5 38 O O H Cu 6 4-MePhSCu (10) 34 iPr Li Ph 7 CuCN (5) 58 N 8 CuCN (2) 73 � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 17 of 22 11/25/2016
Substrate Scope � R 2 R 2 O R 2 Li, CuCN POCl 3 R 1 CN R 1 N R 1 NC THF Et 3 N H then i- PrOCHO Pr NC NC Ph NC Ph NC Ph NC Ph 70% 73% 58% 61% 68% NC NC NC NC BnO MeO Cl 60% 66% 45% 69% NC NC NC NC NC CN Ph N TBSO 53% 41% 55% 54% 40% � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 18 of 22 11/25/2016
Less Successful Substrate Scope � R 2 R 2 O R 2 Li, CuCN POCl 3 R 1 CN R 1 N R 1 NC THF Et 3 N H then i- PrOCHO Pr NC NC NC Ph NC Ph NC NC Ph NC Ph NC Ph N S 70% 73% 58% 61% 68% 27% 31% 31% NC NC NC NC Hexyl BnO MeO Cl 60% 66% 45% 69% NC Ph NC N 0 % 36% NC NC NC NC NC CN Ph N TBSO 53% 41% 55% 54% 40% � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 19 of 22 11/25/2016
[4+2] Cycloaddition � R CuMeSal NC PhMe, 110 o C R R Ph OMe Ph MeO 41% 54% 69% 71% Ph NC Ph NC 1,3 shift Unreactive with the folloing in catalyst free reaction H H NC 2 NC NC Cu O Cu NC CN O OEt Ph -2HCN � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 20 of 22 11/25/2016
[4+2] Cycloaddition � R CuMeSal NC PhMe, 110 o C R R Ph OMe Ph MeO 41% 54% 69% 71% Ph NC Ph NC 1,3 shift H H NC 2 NC NC Cu Cu -2HCN � Angew. Chem. Int. Ed. 2016 , 55, 14770-14773 . � � Mike Frasso @ Wipf Group Page 21 of 22 11/25/2016
Conclusion � • A new preparation of vinyl isonitriles was developed • Advantages: one-pot process, fairly simple to perform, no E / Z mixtures • Disadvantages: only primary alkyl lithium reagent are effective, typical functional group/moisture sensitivity Mike Frasso @ Wipf Group Page 22 of 22 11/25/2016
Recommend
More recommend