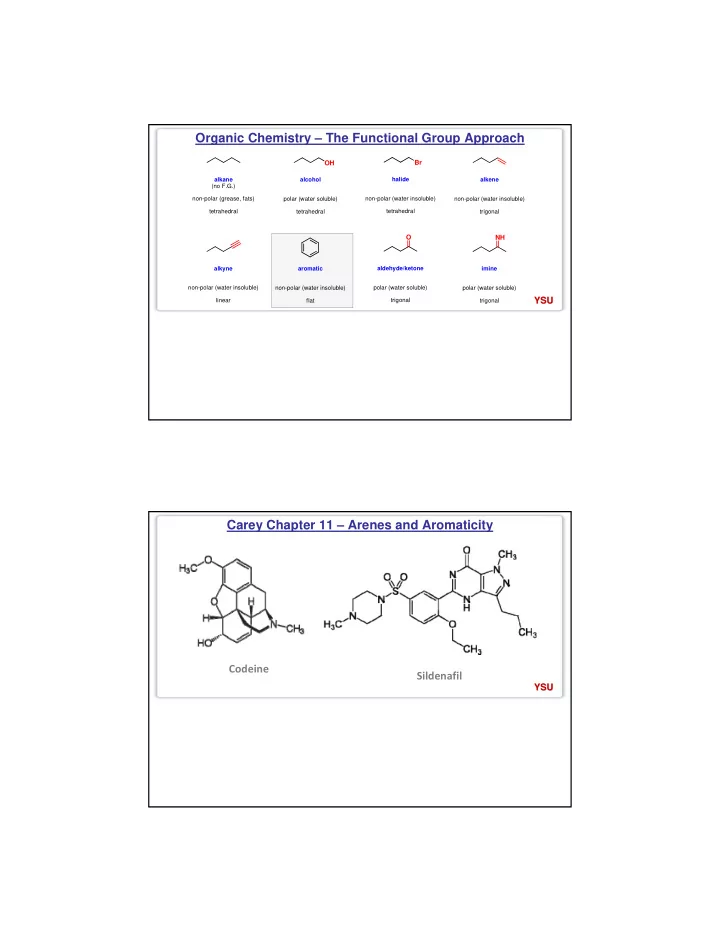

Organic Chemistry – The Functional Group Approach Br OH alkane alcohol halide alkene (no F.G.) non-polar (grease, fats) polar (water soluble) non-polar (water insoluble) non-polar (water insoluble) tetrahedral tetrahedral tetrahedral trigonal O NH aldehyde/ketone alkyne aromatic imine non-polar (water insoluble) non-polar (water insoluble) polar (water soluble) polar (water soluble) YSU YSU linear flat trigonal trigonal Carey Chapter 11 – Arenes and Aromaticity Codeine Sildenafil YSU YSU
11.1 – Increasing Unsaturation in 6-Membered Rings YSU YSU 11.2 – Evidence of Structure for Benzene Kekule (1866) – two rapidly interconverting isomers? all C ‐ C bonds are the same length, all H’s are equivalent YSU YSU
11.2 – Evidence of Structure for Benzene Robinson (1920) ‐ the two Kekule forms are resonance contributors all C ‐ C bonds are the same length, all H’s are equivalent YSU YSU 11.2 – Evidence of Structure for Benzene Robinson depiction : “ Aromatic Sextet ” all C ‐ C bonds are the same length, all H’s are equivalent YSU YSU
11.4 Resonance energy of benzene as estimated from heats of hydrogenation Figure 11.2 Benzene is a lot more stable than “cyclohexatriene” YSU YSU 11.5 – The s bonds (a), the delocalized p system (b), and the electrostatic potential map (c) of benzene Figure 11.3 i.e. each carbon experiences the same electron density, the six pi electrons are delocalized over the entire molecule YSU YSU
11.6 – The molecular orbitals of benzene arranged in order of increasing energy Figure 11.4 YSU YSU Figure 11.4 YSU YSU
11.7 – Nomenclature of Substituted Benzenes Many have common names, however IUPAC systematic names often easier to work out YSU YSU 11.7 – Nomenclature of Disubstituted Benzenes Br CH 3 F Br CH 3 F NH 2 NO 2 CH 3 Br CH 3 CH 2 H 3 CH 3 Can use numbering or o , m , p nomenclature systems YSU YSU
Not Covering 11.8 11.9 11.11 YSU YSU 11.12 – Free-Radical Halogenation of Alkylbenzenes H H C C H H = 91 kcal/mol + H H H H H C C H + H H = 88 kcal/mol H H H H H C H H C H H = 85 kcal/mol + YSU YSU
11.12 – Free-Radical Halogenation of Alkylbenzenes H H H C H H Br C Br 2 ( + HBr ) CCl 4 , 80 o C 71% yield Figure 11.9 YSU YSU 11.13 – Oxidation of Alkylbenzenes YSU YSU
11.14 – Nucleophilic Substitution in Benzylic Halides S N 2 applies with good nucleophiles on 1 o and 2 o carbons S N 1 applies with weak nucleophiles – good carbocation E2 competes with more basic nucleophiles on 2 o and 3 o YSU YSU 11.15 – Preparation of Alkenylbenzenes YSU YSU
11.16 – Addition to Alkenylbenzenes YSU YSU Not Covering 11.17 11.18 YSU YSU
11.19 – Hückel’s Rule http://redandr.ca/vm3/Heme.jpg YSU YSU 11.19 – Hückel’s Rule S N O Aromatic = 4n+2 electrons and flat system YSU YSU
11.19 – Hückel’s Rule Figure 11.12 YSU YSU Not Covering 11.20 YSU YSU
11.21 – Aromatic Ions Cation is relatively easy to form: 4n + 2 = 6 system capable of being flat YSU YSU Figure 11.13 11.21 – Aromatic Ions p K a of acid is ~16 since anion is aromatic: 4n + 2 = 6 system capable of being flat YSU Figure 11.14 YSU
11.23 – Heterocyclic Aromatic Compounds – Hückel’s Rule .. N N .. H pyrrole YSU pyridine YSU Figure 11.15
Recommend
More recommend