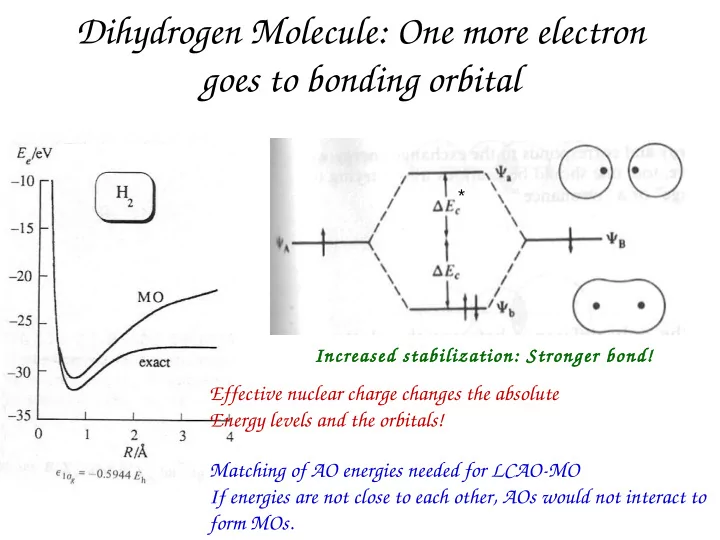

Dihydrogen Molecule: One more electron goes to bonding orbital * Increased stabilization: Stronger bond! Effective nuclear charge changes the absolute Energy levels and the orbitals! Matching of AO energies needed for LCAO-MO If energies are not close to each other, AOs would not interact to form MOs.
Energies of H 2 + , H 2 , He 2 + , He 2 He 2 + He 2
Matching of AO energies for MO ϕ ± = ψ ± ψ Z-axis c c 1 1 2 2 S=0 P X/Y +s P Z + s Both symmetry and energy S>0 matching is required for MO Valence electrons are most important for bonding • Due to large difference in energy of 1s(H) and 1s(F), LCAO-MO for both 1S is not feasible in HF. • Rather only 2Pz(F) [NOT 2Px/y(F)] and 1S(H) form a σ -bond.
Electron Density Maps/Contours
MO Contours electron density maps 2 σ and 2 σ∗ Li 2 : core 1 σ H 2 3 σ and 1 π Li 2 : core 1 σ∗ 1 π∗ Li 2 : Valence 2 σ Total HOMO : Highest Occupied Molecular Orbital Li 2 : Total O 2 molecule LUMO : Lowest Unoccupied Molecular Orbital
Expected MO and Energies for N 2 Are these MO and correct energy level diagram for N 2 ? There is a problem! Spectroscopy says NO!
Actual MO and Energy Diagram for N 2 Nature 2004 vol 432 867 Mixing of 2S and 2P orbital occur because of small energy gap between them. 2s and 2p electrons feels not so different effective nuclear charge.
S-P Mixing in Atomic Orbitals ψ = ψ ± ψ C1 and C2 not equal – SPz c c s p ± 1 2 2 2 z In the mix orbital: S and P Z different contributions 2S and 2P AOs MIX due to less energy gap 2s and 2p e feels not so different Z eff . Mixed states can form MOs
s-p Mixing: B 2 magnetism confirms it! Incorrect! Boron is paramagnetic. This can only happen if the two electrons with parallel spin are in the π -orbitals π -bonding energies lower than σ *?
Recommend
More recommend