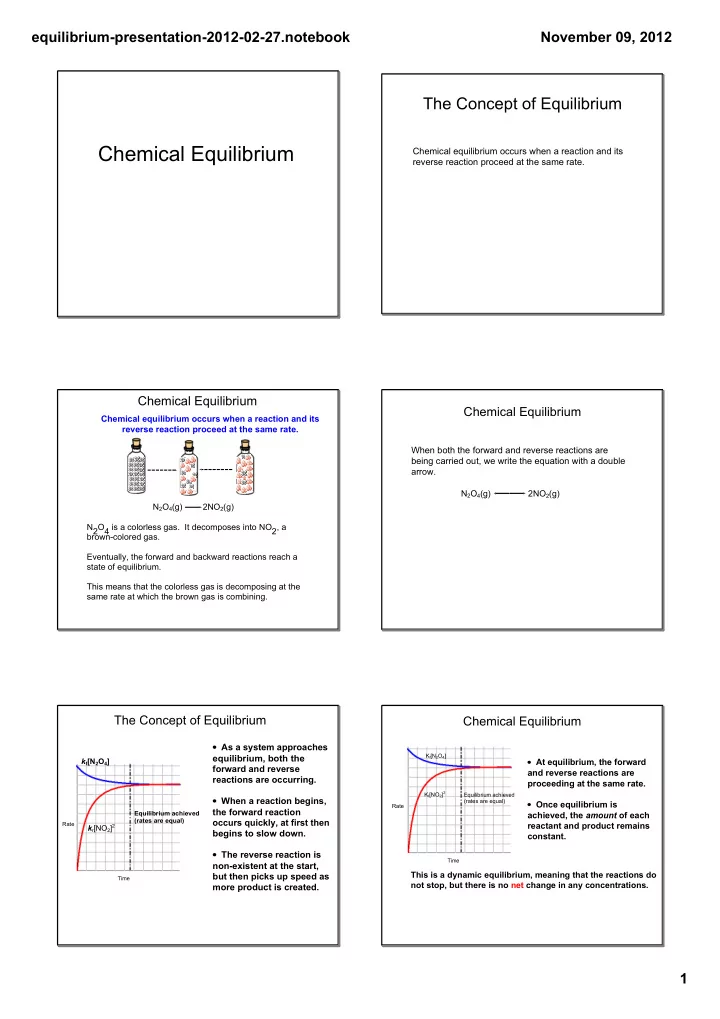

equilibriumpresentation20120227.notebook November 09, 2012 The Concept of Equilibrium Chemical Equilibrium Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate. Chemical Equilibrium Chemical Equilibrium Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate. When both the forward and reverse reactions are being carried out, we write the equation with a double arrow. N 2 O 4 (g) 2NO 2 (g) N 2 O 4 (g) 2NO 2 (g) N 2 O 4 is a colorless gas. It decomposes into NO 2 , a browncolored gas. Eventually, the forward and backward reactions reach a state of equilibrium. This means that the colorless gas is decomposing at the same rate at which the brown gas is combining. The Concept of Equilibrium Chemical Equilibrium • As a system approaches K f [N 2 O 4 ] equilibrium, both the • At equilibrium, the forward k f [N 2 O 4 ] forward and reverse and reverse reactions are reactions are occurring. proceeding at the same rate. K f [NO 2 ] 2 Equilibrium achieved • When a reaction begins, (rates are equal) • Once equilibrium is Rate the forward reaction Equilibrium achieved achieved, the amount of each (rates are equal) occurs quickly, at first then Rate reactant and product remains k r [NO 2 ] 2 begins to slow down. constant. • The reverse reaction is Time nonexistent at the start, This is a dynamic equilibrium, meaning that the reactions do but then picks up speed as Time not stop, but there is no net change in any concentrations. more product is created. 1
equilibriumpresentation20120227.notebook November 09, 2012 Chemical Equilibrium Chemical Equilibrium It is important to note that equilibrium can be achieved K f [N 2 O 4 ] Once equilibrium is achieved, two regardless of whether you start with reactants or products, important conditions apply: as long as there is sufficient material to get both processes going. (1) the forward and reverse reactions K f [NO 2 ] 2 Equilibrium achieved N 2 (g) + 3H 2 (g) 2NH 3 (g) (rates are equal) are proceeding at the same rate . Rate (2) the amount of each reactant and product remains constant. Time (This does not imply that the amount of reactants = amount of products.) Chemical Equilibrium Chemical Equilibrium N 2 (g) + 3H 2 (g) 2NH 3 (g) N 2 (g) + 3H 2 (g) 2NH 3 (g) Regardless of the starting material, however, the same In this graph, observe that the In this graph, observe that the equilibrium concentrations of all three substances will only substance initially only substances initially eventually be reached. present is ammonia; there is present are nitrogen and no nitrogen and hydrogen at hydrogen; there is no ammonia the start. at the start. 1 At equilibrium, 2 Which of the following statements is NOT true _______________________. about a reversible reaction at equilibrium? A A all chemical reactions have ceased reactant and product concentrations are constant the rates of forward and reverse reactants and products remain in B B reactions are equal dynamic equilibrium the rate constants of the C reactant concentrations are forward and reverse reactions C equal to product concentrations are equal the value of the equilibrium the forward reaction occurs at D D constant is 1 the same rate as the reverse reaction E the limiting reagent has been consumed 2
equilibriumpresentation20120227.notebook November 09, 2012 3 To achieve equilibrium, you must have only 4 Consider the equilibrium A + 3B <> 2C. reactants to start with. Equilibrium can be reached if you start with: A only A True B only B False C only C 5 Consider the equilibrium A + 3B <> 2C. 6 Consider the equilibrium Equilibrium cannot be reached if you start with: 2 SO 2 (g) + O 2 (g) 2 SO 3 (g). Equilibrium cannot be established when A A & B _________is/are in the container. B only B C only C A 0.5 mol SO 2 and 0.5 mol O 2 B 0.5 mol SO 3 C 0.5 mol O 2 and 0.5 mol SO 3 D 0.5 mol SO 2 E 0.5 mol SO 2 and 0.5 mol SO 3 The Equilibrium Constant The Equilibrium Constant • Recall that the definition of chemical equilibrium is when the The ratio of the rate constants is known as the rate of the forward reaction equals the rate of the reverse equilibrium constant, K. The ratio simplifies to a reaction. ratio of products to reactants. N 2 O 4 (g) <> 2NO 2 (g) • Therefore, at equilibrium, we can set the two rate laws equal: k f [NO 2 ] 2 K eq = = Rate of forward reaction = Rate of reverse reaction k r [N 2 O 4 ] k f [N 2 O 4 ] = k r [NO 2 ] 2 The value of K eq is constant for a specific reversible • Rewriting this, it becomes reaction at a certain temperature. Like rate k f [NO 2 ] 2 constants, an equilibrium constant, K eq will only = k r [N 2 O 4 ] change when there is a change in temperature. 3
equilibriumpresentation20120227.notebook November 09, 2012 The Equilibrium Constant The Equilibrium Constant The equilibrium constant expression can be written from a Pure solids and liquids are not included in the balanced chemical equation calculation of the equilibrium constant. You can take their concentration to be 1, and not include them. aA + bB cC + dD That's because their concentrations don't change during according to the general format: a reaction. [C] c [D] d Molarity = density / molar mass K c = K = [A] a [B] b = (g/L) / (g/mol) = mol/L Since neither density nor molar mass varies for a pure liquid or pure solid, we take their molarities to remain constant. The Equilibrium Constant The Equilibrium Constant The equilibrium constant for the reaction is The equilibrium constant for the reaction is 2Cl 2 (g) + 2H 2 O (g) CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) 4HCl(g) + O 2 (g) [HCl] 4 [O 2 ] [CO 2 ] K = K = [CH 4 ] [O 2 ] 2 [Cl 2 ] 2 [ H 2 O] 2 The Equilibrium Constant 7 The equilibriumconstant expression depends on the __________ of the reaction. The equilibrium constant for the reaction is A stoichiometry (CH 3 ) 4 Sn(s) (CH 3 ) 4 Sn(g) B mechanism C stoichiometry and mechanism D the quantities of reactants and products initially present E temperature K = [(CH 3 ) 4 Sn] 4
equilibriumpresentation20120227.notebook November 09, 2012 What Does the Value of K Mean? 8 Which one of the following will change the value of an equilibrium constant? • If K >1, the reaction is product A changing temperature favored ; product predominates Reactants at equilibrium. adding other substances that do not react with any of Products B the species involved in the equilibrium K > 1 C varying the initial concentrations of reactants • If K<1, the reaction is D varying the initial concentrations of products reactantfavored ; reactant E changing the volume of the reaction vessel predominates at Products equilibrium. Reactants K < 1 9 For a reaction at equilibrium, Equilibrium a value of K= 1 x 10 8 means that The direction from which equilibrium is achieved (starting with all products or all reactants) doesn't A reactants are favored matter. B products are favored C the reaction is spontaneous It is only a convention that we calculate K by dividing the concentrations of products over D the reaction is endothermic reactants, since the reaction proceeds both ways. Unless specified, read the equation left to right Left side reactants; right side products. But that convention makes it easy to understand the meaning of K eq . 10 For a reaction at equilibrium, a 11 X <> Y + Z value of K = 4 x 10 12 means that What is the equilibrium constant expression for the reaction above? A reactants and products are present in equal amounts the forward reaction is occurring faster than K = [X] / [Y] +[Z] A B the reverse reaction B K = [X] / [Y] [Z] the reverse reaction is occurring faster than C the forward reaction C K = [X] / [Y] [Z] D reactants are favored D K = [Y] + [Z] / [X] E products are favored K = [Y] [Z] / [X] E 5
equilibriumpresentation20120227.notebook November 09, 2012 13 A + 2B <> C 12 X <> Y + Z Calculate the value of the equilibrium Calculate the value of the equilibrium constant, K, for the reaction above if the equilibrium concentrations are: constant, K, for the reaction above if the [A] = 0.1 M, [B] = 0.2 M, [C] = 0.4 M equilibrium concentrations are: [X] = 0.3 M, [Y] = 0.5 M, [Z] = 1.2 M 14 2A + B <> C 15 X <> 2Y + Z The value of the equilibrium constant, K, The value of the equilibrium constant, K, for the reaction above at 400 K is 100. for the reaction above at 350 K is 0.5. Calculate the equilibrium concentration Calculate the equilibrium concentration of C, given these equilibrium of X, given these equilibrium concentrations at 400 K: concentrations at 400 K: [A] = 0.1 M, [B] = 0.4 M [Y] = 2 M, [Z] = 1 M [C] = ____ M [X] = ____ M The Equilibrium Constant 16 What is the equilibrium expression for K p for Consider the generalized reaction the reaction given. aA + bB cC + dD 2 O 3 (g) 3 O 2 (g) Since pressure is proportional to concentration for gases in a closed system, the equilibrium expression can also be written in terms of pressure (P A ) a (P B ) b 3 P O 2 / 2 P O3 A K p = B 2P O3 / 3 P O2 (P C ) c (P D ) d C 3 P O3 / 2 P O2 where (P A ) a (P B ) b P c = partial pressure of gaseous substance C D (P O3 ) 2 / (P O2 ) 3 K p = P A = partial pressure of gaseous substance A (P C ) c (P D ) d (P O2 ) 3 / (P O3 ) 2 E and so forth for B and D Recall that P A = X A (P tot ) where X A is the mole fraction of the gas and P tot is total pressure. 6
Recommend
More recommend