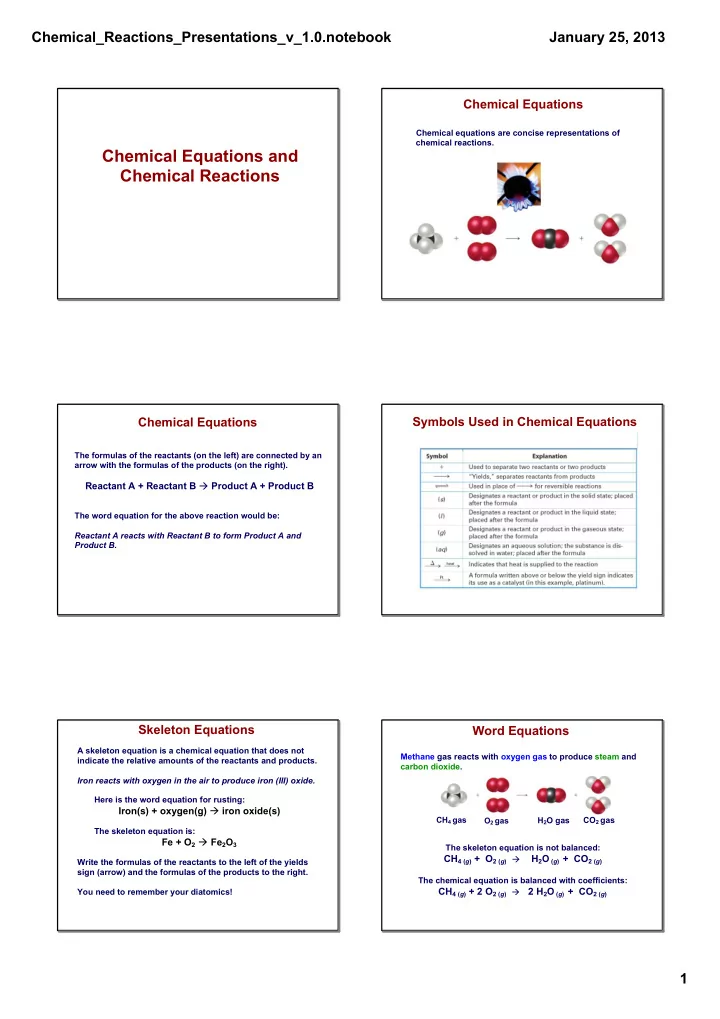

Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 Chemical Equations Chemical equations are concise representations of chemical reactions. Chemical Equations and Chemical Reactions Symbols Used in Chemical Equations Chemical Equations The formulas of the reactants (on the left) are connected by an arrow with the formulas of the products (on the right). Reactant A + Reactant B � Product A + Product B The word equation for the above reaction would be: Reactant A reacts with Reactant B to form Product A and Product B. Skeleton Equations Word Equations A skeleton equation is a chemical equation that does not Methane gas reacts with oxygen gas to produce steam and indicate the relative amounts of the reactants and products. carbon dioxide . Iron reacts with oxygen in the air to produce iron (III) oxide. Here is the word equation for rusting: Iron(s) + oxygen(g) � iron oxide(s) CH 4 gas H 2 O gas CO 2 gas O 2 gas The skeleton equation is: Fe + O 2 � Fe 2 O 3 The skeleton equation is not balanced: CH 4 ( g ) + O 2 ( g ) � H 2 O ( g ) + CO 2 ( g ) Write the formulas of the reactants to the left of the yields sign (arrow) and the formulas of the products to the right. The chemical equation is balanced with coefficients: CH 4 ( g ) + 2 O 2 ( g ) � 2 H 2 O ( g ) + CO 2 ( g ) You need to remember your diatomics! 1
Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 In the reaction 2 In the reaction 1 CH 4 ( g ) + O 2 ( g ) � H 2 O ( g ) + CO 2 ( g ) CH 4 ( g ) + O 2 ( g ) � H 2 O ( g ) + CO 2 ( g ) the products are: the products are: A solids oxygen and water A liquids B carbon dioxide and water B gases C oxygen and methane C D dissolved in water (aqueous) methane and carbon dioxide D cannot be determined E Word Equations to Chemical Equations Word Equations to Chemical Equations Solid potasium chlorate decomposes in air to produce solid Write the word equation, then the skeleton equation for potassium chloride and oxygen gas. this reaction: Aluminum sulfate reacts with calcium chloride to The "word" equation is: produce calcium sulfate and aluminum chloride potasium chlorate (s) � potassium chloride (s) + oxygen (g) The unbalanced "skeleton" equation is: KClO 3(s) � KCl (s) + O 2(g) Balancing Chemical Equations Law of Conservation of Mass To write a balanced chemical equation, first write the skeleton equation. Then use coefficients to balance the “We may lay it down as an incontestable axiom that, in all equation so that it obeys the law of conservation of mass. the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the This is a balanced equation for making a bicycle. The experiment. Upon this principle, the whole art of numbers are called coefficients—small whole numbers that performing chemical experiments depends.” are placed in front of the formulas in an equation in order to balance it. Antoine Lavoisier, 1789 2
Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 Balancing Chemical Equations Balancing Chemical Equations CH 4 ( g ) + 2 O 2 ( g ) � CO 2 ( g ) + 2 H 2 O ( g ) CH 4 ( g ) + 2 O 2 ( g ) � CO 2 ( g ) + 2 H 2 O ( g ) Products appear on the Reactants appear on the right side of the equation. left side of the equation. Coefficients are inserted to balance the equation. The states of the reactants and products are written in 1's typically are not written. parentheses to the right of each compound. Subscripts and Coefficients How many oxygen atoms are in 3 one formula unit of calcium nitrate? (First, write the formula for calcium nitrate.) Subscripts tell the number of atoms of each element in a molecule. Coefficients tell the number of representative particles (atoms, molecules, or formula units). Balancing Chemical Equations 4 How many nitrogen atoms are in one formula unit of H 2 + Cl 2 � HCl H 2 + Cl 2 � 2HCl ammonium sulfate? balanced unbalanced 1 2 2 2 2 2 2 1 3
Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 Balancing Chemical Equations 5 When the following Write a balanced chemical equation for this reaction. equation is balanced, the (First write a skeleton equation, then balance it) coefficients are chlorine + sodium bromide � bromine + sodium chloride Na + O 2 � Na 2 O Enter ### on your responder (112, etc) 7 When the following equation is balanced, the 6 When the following coefficients are equation is balanced, the coefficients are HgO � Hg + O 2 Al + ZnCl 2 � Zn + AlCl 3 8 When the following 9 When the following equation is balanced, the equation is balanced, the coefficients are coefficients are NH 3 + O 2 � NO 2 + H 2 O NaCl + CaI 2 � NaI + CaCl 2 4
Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 Reaction Types 10 When the following equation is balanced, the coefficients are There are 5 major types of chemical reactions that we will study this year. • Combination Al(NO 3 ) 3 + Na 2 S � Al 2 S 3 + NaNO 3 • Decomposition • Combustion • Single replacement • Double replacement Combination Reactions 11 Which of the following is a combination reaction? A H 2 O � H 2 +O 2 Two or more Na + Cl 2 � NaCl B substances AgNO 3 + BaSO 4 � Ag 2 SO 4 + Ba(NO 3 ) 2 C react to form C 3 H 8 + O 2 � H 2 O + CO 2 one product. D Examples: • 2 Mg (s) + O 2(g) � 2 MgO (s) • N 2(g) + 3 H 2(g) � 2 NH 3(g) • C 3 H 6(g) + Br 2(l) � C 3 H 6 Br 2(l) 12 Which of the following is a Decomposition Reactions decomposition reaction? One substance A H 2 O � H 2 +O 2 breaks down into B Na + Cl 2 � NaCl two or more AgNO 3 + BaSO 4 � Ag 2 SO 4 + Ba(NO 3 ) 2 substances. C C 3 H 8 + O 2 � H 2 O + CO 2 D Examples: • CaCO 3 (s) � CaO (s) + CO 2 (g) • 2 KClO 3 (s) � 2 KCl (s) + 3 O 2 (g) • 2 NaN 3 (s) � 2 Na (s) + 3 N 2 (g) 5
Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 Combustion Reactions Combustion Reactions These are generally rapid reactions that Oxygen is always a reactant in combustion reactions. produce a flame. Examples: Most often involve • CH 4 (g) + 2 O 2 (g) � CO 2 (g) + 2 H 2 O (g) hydrocarbons • C 3 H 8 (g) + 5 O 2 (g) � 3 CO 2 (g) + 4 H 2 O (g) reacting with oxygen in the air. However, other elements may also undergo combustion: O 2 is always one of Mg (s) + O 2 (g) � MgO (s) the reactants. N 2 (g) + 2 O 2 (g) � 2 NO 2 (g) Examples: These last two may also be classified as combination • CH 4 (g) + 2 O 2 (g) � CO 2 (g) + 2 H 2 O (g) reactions. • C 3 H 8 (g) + 5 O 2 (g) � 3 CO 2 (g) + 4 H 2 O (g) 13 Which of the following is a Combustion Reactions combustion reaction? Combustion reactions are easily identified by H 2 O � H 2 +O 2 A oxygen as a reactant. However, the products of a Na + Cl 2 � NaCl B combustion reaction may vary depending on how much oxygen is available. AgNO 3 + BaSO 4 � Ag 2 SO 4 + Ba(NO 3 ) 2 C C 3 H 8 + O 2 � H 2 O + CO 2 D • Complete combustion products are carbon di oxide and water • Incomplete combustion products are carbon mono xide and water Single Replacement Reactions Single Replacement Reactions Single replacement Single replacement reactions can also involve reactions occur between nonmetals, specifically, halogens. an element and a compound. In these reactions, one halogen will displace the halogen from the compound. Usually, a pure metal will displace the metal from F 2 + NaCl � Cl 2 + NaF the compound. M + XY � X + M Y 6
Chemical_Reactions_Presentations_v_1.0.notebook January 25, 2013 Which of the following is a 14 Single Replacement Reactions single replacement reaction? In this reaction, A H 2 O � H 2 +O 2 copper metal Na + Cl 2 � NaCl B displaces silver AgNO 3 + Ba � Ag + Ba(NO 3 ) 2 C ions in the C 3 H 8 + O 2 � H 2 O + CO 2 compound. D The reverse reaction, however, does not occur. Cu (s) + 2 AgNO 3 (aq) � Cu(NO 3) ) 2 (aq) + 2 Ag (s) Activity Series Activity Series Single replacement reactions only occur if the pure metal Note that hydrogen has a higher reactivity than the metal in the compound. appears on this list. Hydrogen can be The reactivity/activity of the metals is provided in a table, displaced from water with the more reactive metals higher on the list. and from some acids by metals that are If a pure metal is higher on the list than the metal compound above it on the Activity it's to react with, the reaction will takes place. Series. Such reactions yield hydrogen gas as If the pure metal is below the metal compound, the reaction a product. will not occur. Activity Series Activity Series Lithium metal will replace calcium metal from its You have a copy of the activity series on the compound such as CaCl 2 since Li is more reactive back of your periodic table. than Ca. Li + CaCl 2 � Ca + 2 LiCl yes! Remember, Lithium (Li) is the most reactive and will replace all other metals. But the reverse reaction will not occur. Gold (Au) is the least reactive and will not replace any other metals (on the list). Ca + LiCl � NO! Ca is less active than Li so it cannot displace Li. 7
Recommend
More recommend