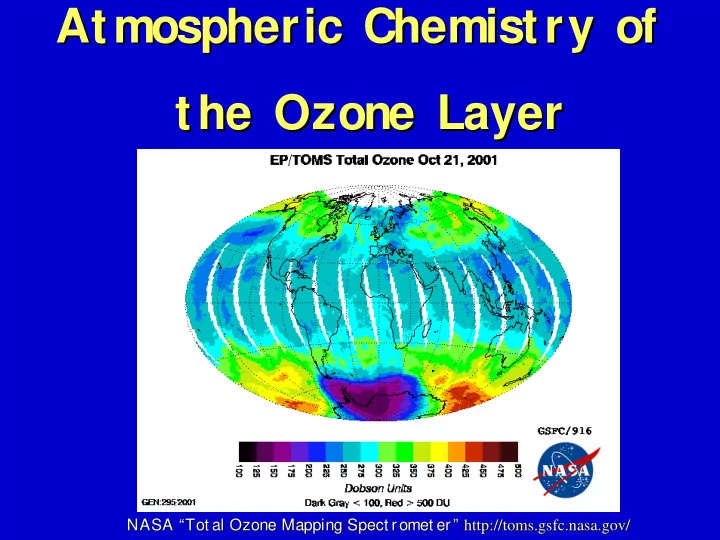

Atmospheric Chemistry of Atmospheric Chemistry of the Ozone Layer the Ozone Layer NASA “Tot al Ozone Mapping Spect romet er” NASA “Tot al Ozone Mapping Spect romet er” http://toms. http://toms.gsfc gsfc. .nasa nasa. .gov gov/ /
Levels of Atmospheric Ozone have been Dropping Levels of Atmospheric Ozone have been Dropping EPA EPA - - ht t p:/ / ht t p:/ / www.epa.gov/ docs/ ozone/ science/ arosa.ht ml
Decreasing Level of atmospheric ozone is harmf ul Decreasing Level of atmospheric ozone is harmf ul There has been an increase in the number There has been an increase in the number of cases of skin cancer and cataracts of cases of skin cancer and cataracts Evidence of damage to plant and marine lif e Evidence of damage to plant and marine lif e
What is ozone? What is ozone? Where in the atmosphere is it f ound? Where in the atmosphere is it f ound? What is its purpose in the atmosphere? What is its purpose in the atmosphere? What is its chemistry? What is its chemistry? Why are levels of atmospheric ozone dropping? Why are levels of atmospheric ozone dropping? Finally, what is the Ozone Hole? Finally, what is the Ozone Hole?
Structure of Ozone Structure of Ozone O Atoms O Atoms O 3 O 3
Where is ozone f ound in the atmosphere ? Where is ozone f ound in the atmosphere ? NASA NASA Goddard Goddard Space Flight Cent er Space Flight Cent er Note, higher concentration in stratosphere, compared Note, higher concentration in stratosphere, compared with troposphere with troposphere
Role of Ozone Role of Ozone Solar Flux Solar Flux Chemical Kinetics and Photochemical Data f or Use in Chemical Kinetics and Photochemical Data f or Use in Stratospheric Modeling Stratospheric Modeling - - JPL Publication97 JPL Publication97- - 4 4
Role of Ozone Role of Ozone Absorption Spectrum of Ozone Absorption Spectrum of Ozone
Role of Ozone Role of Ozone “The Ozone Depletion Phenomenon”, Beyond Discovery, “The Ozone Depletion Phenomenon”, Beyond Discovery, National Academy of Sciences National Academy of Sciences
Role of Ozone Role of Ozone UV A (~400 to 350 nm nm) not absorbed by earth’s ) not absorbed by earth’s UV A (~400 to 350 atmosphere atmosphere UV B (~ 350 to 270 nm nm) partially absorbed by ) partially absorbed by UV B (~ 350 to 270 earth’s atmosphere earth’s atmosphere UV C (~270 to 150 nm nm) completely absorbed by ) completely absorbed by UV C (~270 to 150 earth’s atmosphere earth’s atmosphere UV B is harmf ul to lif e on earth UV B is harmf ul to lif e on earth
How is ozone production and destruction? How is ozone production and destruction? Chapman mechanism Chapman mechanism ν - ν ν ν h ν ν ν ν O 2 + h - > O + O > O + O O 2 + O + O 2 + M- - > O > O 3 + M O + O 2 + M 3 + M ν ν′ ν ν ′ - ′ ′ ′ h ν ν ν ν ′ ′ ′ O 3 + h - > O + O > O + O 2 O 3 + 2 O + O 3 - > 2 O > 2 O 2 O + O 3 - 2 “Ozone: What is it and why do we care “Ozone: What is it and why do we care about it?”, NASA Facts, Goddard Goddard Space Space about it?”, NASA Facts, Flight Center Flight Center
Kinetics of Chapman Mechanism Kinetics of Chapman Mechanism ν ν ν - ν h ν ν ν ν O 2 + h - > O + O > O + O k 1 O 2 + k 1 O + O 2 + M- - > O > O 3 + M k 2 O + O 2 + M 3 + M k 2 ν ν ν ν′ ′ ′ - ′ ′ h ν ν ν ν ′ ′ ′ O 3 + h - > O + O > O + O 2 k 3 O 3 + k 2 3 O + O 3 - > 2 O > 2 O 2 k 4 O + O 3 - k 2 4 Rate of f ormation of O and O 3 Rate of f ormation of O and O 3 d[O]/ dt dt = 2k = 2k 1 [O 2 ]- - k k 2 [O][O 2 ][M] + k 3 [O 3 ] - - k k 4 [O][O 3 ] d[O]/ 1 [O 2 ] 2 [O][O 2 ][M] + k 3 [O 3 ] 4 [O][O 3 ] d[O 3 ]/ dt dt = k = k 2 [O][O 2 ][M] - - k k 3 [O 3 ]- - k k 4 [O][O 3 ] d[O 3 ]/ 2 [O][O 2 ][M] 3 [O 3 ] 4 [O][O 3 ] Steady- - State Approximation State Approximation Steady d[O]/ dt dt = d[O = d[O 3 ]/ dt dt = 0 = 0 d[O]/ 3 ]/
Kinetics of Chapman Mechanism Kinetics of Chapman Mechanism d[O 3 ]/ dt dt = k = k 2 [O][O 2 ][M] - - k k 3 [O 3 ]- - k k 4 [O] [O [O 3 ]=0 =0 d[O 3 ]/ 2 [O][O 2 ][M] 3 [O 3 ] 4 [O] 3 ] k 2 [O][O 2 ][M]={ k 3 +k 4 [O] } [O [O 3 ] k 2 [O][O 2 ][M]={ k 3 +k 4 [O] } 3 ] k 2 [O][O 2 ][M]/ { k 3 +k 4 [O] } = [O [O 3 ] k 2 [O][O 2 ][M]/ { k 3 +k 4 [O] } = 3 ]
Kinetics of Chapman Mechanism Kinetics of Chapman Mechanism Can re- - write [O write [O 3 ] as: Can re 3 ] as: [O 3 ] = k = k 2 [O][O 2 ][M]/ { k 3 +k k 4 [O] } } [O 3 ] 2 [O][O 2 ][M]/ { k 3 + 4 [O] (Divide by k k 4 [O] ) ) (Divide by 4 [O] [O 3 ] = k 2 [O 2 ][M]/ k 4 k 3 /(k 4 [O]) + 1
Kinetics of Chapman Mechanism Kinetics of Chapman Mechanism Since the rate constants and concentration of Since the rate constants and concentration of species are known, can show that: species are known, can show that: k 3 [O 3 ] = k 2 [O 2 ][M]/ k 4 k 4 [O] >> 1 + + k 3 /(k 4 [O]) + 1 Hence, Hence, [O 3 ] ≈ k 2 [O 2 ][M][O] k 3
Kinetics of Chapman Mechanism Kinetics of Chapman Mechanism [O 3 ] depends on rate of reaction 2 [O 3 ] depends on rate of reaction 2 [O 3 ] ≈ k 2 [O 2 ][M][O] and the intensity of light (k 3 ) k 3 and the intensity of light (k 3 ) Reaction 2 is slow (termolecular termolecular); makes ozone ); makes ozone Reaction 2 is slow ( “vulnerable” to ozone- - depleting reactions depleting reactions “vulnerable” to ozone ν ν - ν ν h ν ν ν ν O 2 + h - > O + O > O + O k 1 O 2 + k 1 O + O 2 + M- - > O > O 3 + M k 2 O + O 2 + M 3 + M k 2 ν ν′ ν ν ′ - ′ ′ ′ h ν ν ν ν ′ ′ ′ O 3 + h - > O + O > O + O 2 k 3 O 3 + k 2 3 O + O 3 - > 2 O > 2 O 2 k 4 O + O 3 - k 2 4
Competing Reactions Competing Reactions HO x cycle HO x cycle H, OH and HO 2 species f ormed by H, OH and HO 2 species f ormed by reaction of excited O atoms with H- - reaction of excited O atoms with H containing atmospheric species like H 2 O containing atmospheric species like H 2 O and CH 2 and CH 2 ν ν ν ν′ ′ - ′ ′ ′ h ν ν ν ν ′ ′ ′ O 3 + h - > O + O > O + O 2 O 3 + 2 O + H 2 O - - > OH + OH > OH + OH O + H 2 O O + CH 4 - > CH > CH 3 + OH O + CH 4 - 3 + OH ν - ν ν ν h ν ν ν ν H 2 O + h - > H + OH > H + OH H 2 O +
Reactions of HO HO x species with O 3 Reactions of x species with O 3 OH + O 3 - > HO > HO 2 + O 2 OH + O 3 - 2 + O 2 HO 2 + O - - > OH + O > OH + O 2 HO 2 + O 2 Net Reaction Net Reaction O + O 3 - > 2O > 2O 2 O + O 3 - 2 “Ozone Depletion” “Ozone Depletion”
Competing Reactions Competing Reactions NO x Cycle NO x Cycle NOx species are produced during the species are produced during the NOx reaction of O atoms with N 2 O (produced in reaction of O atoms with N 2 O (produced in the soil by bacteria) the soil by bacteria) O + N 2 O - - > 2 NO > 2 NO O + N 2 O
Reactions of NO NO x species with O 3 Reactions of x species with O 3 NO + O 3 - > NO > NO 2 + O 2 NO + O 3 - 2 + O 2 NO 2 + O - - > NO + O > NO + O 2 NO 2 + O 2 Net Reaction Net Reaction O + O 3 - > 2O > 2O 2 O + O 3 - 2 “Ozone Depletion” “Ozone Depletion”
Competing Reactions Competing Reactions ClO x cycle ClO x cycle ClO x species are produced f rom chlorof luorocarbons chlorof luorocarbons ClO x species are produced f rom (CFC’s CFC’s) and methyl chloride ) and methyl chloride ( CFC’s are artif icially produced; methyl chloride is are artif icially produced; methyl chloride is CFC’s a naturally occuring occuring chemical. chemical. a naturally Examples of CFC’s CFC’s : : Freons Freons (CFCl (CFCl 3 , CF 2 Cl 2 ) Examples of 3 , CF 2 Cl 2 ) ν ν - ν ν h ν ν ν ν CCl 2 F 2 + h - > CF > CF 2 Cl + Cl Cl CCl 2 F 2 + 2 Cl + CCl 2 F 2 + O - - > CF > CF 2 Cl + ClO ClO CCl 2 F 2 + O 2 Cl +
Reactions of ClO ClO x species with O 3 Reactions of x species with O 3 Cl + O + O 3 - > > ClO ClO + O + O 2 Cl 3 - 2 ClO + O + O - - > > Cl Cl + O + O 2 ClO 2 Net Reaction Net Reaction O + O 3 - > 2O > 2O 2 O + O 3 - 2 “Ozone Depletion” “Ozone Depletion” 1995 Nobel Prize in Chemistry 1995 Nobel Prize in Chemistry
Consequences of Competing Reactions Consequences of Competing Reactions Catalytic Reactions Catalytic Reactions catalyst intermediate catalyst intermediate Cl + O + O 3 - > > ClO ClO + O + O 2 Cl 3 - 2 ClO + O + O - - > > Cl Cl + O + O 2 ClO 2 catalyst catalyst intermediate intermediate - lower activation energy lower activation energy - E a f or Chapman mechanism = 17. 1 kJ/ mol mol E a f or Chapman mechanism = 17. 1 kJ/ E a f or ClO ClO x reaction = 2. 1 kJ/ mol mol E a f or x reaction = 2. 1 kJ/
Consequences of Competing Reactions Consequences of Competing Reactions Ef f ect of competing reaction on rate of ozone f ormation Ef f ect of competing reaction on rate of ozone f ormation Depleting reactions are NOT independent of each Depleting reactions are NOT independent of each other; in f act all occur simultaneously other; in f act all occur simultaneously NET LOSS OF OZONE NET LOSS OF OZONE
Recommend
More recommend