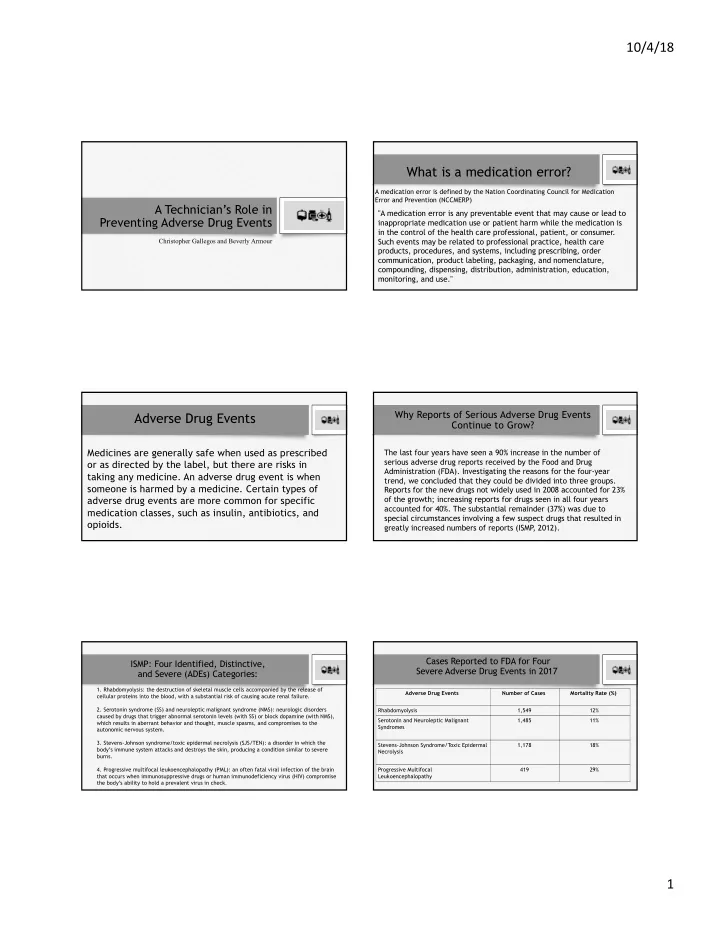

10/4/18 What is a medication error? A medication error is defined by the Nation Coordinating Council for Medication Error and Prevention (NCCMERP) A Technician’s Role in "A medication error is any preventable event that may cause or lead to Preventing Adverse Drug Events inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care Christopher Gallegos and Beverly Armour products, procedures, and systems, including prescribing, order communication, product labeling, packaging, and nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use." Why Reports of Serious Adverse Drug Events Adverse Drug Events Continue to Grow? Medicines are generally safe when used as prescribed The last four years have seen a 90% increase in the number of serious adverse drug reports received by the Food and Drug or as directed by the label, but there are risks in Administration (FDA). Investigating the reasons for the four-year taking any medicine. An adverse drug event is when trend, we concluded that they could be divided into three groups. someone is harmed by a medicine. Certain types of Reports for the new drugs not widely used in 2008 accounted for 23% adverse drug events are more common for specific of the growth; increasing reports for drugs seen in all four years accounted for 40%. The substantial remainder (37%) was due to medication classes, such as insulin, antibiotics, and special circumstances involving a few suspect drugs that resulted in opioids. greatly increased numbers of reports (ISMP , 2012). Cases Reported to FDA for Four ISMP: Four Identified, Distinctive, Severe Adverse Drug Events in 2017 and Severe (ADEs) Categories: 1. Rhabdomyolysis: the destruction of skeletal muscle cells accompanied by the release of Adverse Drug Events Number of Cases Mortality Rate (%) cellular proteins into the blood, with a substantial risk of causing acute renal failure. 2. Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS): neurologic disorders Rhabdomyolysis 1,549 12% caused by drugs that trigger abnormal serotonin levels (with SS) or block dopamine (with NMS), Serotonin and Neuroleptic Malignant 1,485 11% which results in aberrant behavior and thought, muscle spasms, and compromises to the Syndromes autonomic nervous system. 3. Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): a disorder in which the Stevens-Johnson Syndrome/Toxic Epidermal 1,178 18% body’s immune system attacks and destroys the skin, producing a condition similar to severe Necrolysis burns. 4. Progressive multifocal leukoencephalopathy (PML): an often fatal viral infection of the brain Progressive Multifocal 419 29% that occurs when immunosuppressive drugs or human immunodeficiency virus (HIV) compromise Leukoencephalopathy the body’s ability to hold a prevalent virus in check. 1
10/4/18 Suspect Drugs Cases Suspect Drugs Cases Suspect Drugs Cases Suspect Cases Drugs Statins: 264 Analgesics: 86 Atorvastatin 114 Methadone 30 Antidepressant: 284 Antipsychotics: 82 Simvastatin 97 Acetaminophen 24 Rosuvastatin 53 Pregablin 17 *Antipsychotics: 184 Gabapentin 15 Sertraline 63 Aripiprazole 37 Aripiprazole 83 Anticonvulsants: 77 Quetiapine 23 Levetiracetam 64 Venlafaxine 50 Quetiapine 31 Risperidone 19 Lamotrigine 13 Olanzapine 19 Other: 68 Adverse Event: Fluoxetine 35 Olanzapine 14 Paliperidone 18 Metformin 20 Clozapine 12 Daptomycin 15 Strong Signals for Escitalopram 29 Other: 61 Haloperidol 10 Furosemide 12 Antidepressant: 25 Amlodipine 11 Duloxetine 26 Linezolid 17 Sertraline 13 Sacubitril/Valsartan 10 Serotonin Syndrome Venlafaxine 12 *Most of these were secondary to Neuroleptic Malignant Syndrome (NMS) Citalopram 22 Methylphenidate 12 Antineoplastics: 40 Nivolumab 25 Trabectedin 15 Vortioxetine 21 Ondansetron 11 Paroxetine 20 Lithium 11 Adverse Event: Strong Signals for Rhabdomyolysis Bupropion 18 Sodium Oxybate 10 Opioids: 47 Tramadol 37 Tapentadol 10 Suspect Cases Suspect Cases Drugs Drugs Anticonvulsants 210 Antineoplastics 72 Suspect Cases Lamotrigine 128 Nivolumab 23 Carbamazepine 34 Pembrolizumab 20 Drugs Phenytoin 22 Lenalidomide 17 Adverse Event: Strong Adverse Event: Strong Valproic Acid 13 Cobimetinib 12 Signals for Stevens- Natalizumab 154 Levetiracetam 13 Antipsychotics 16 Signals for Progressive Antibiotics/Antifungals 105 Aripiprazole 16 Johnson Syndrome/Toxic Multifocal Rituximab 59 Sulfamethoxazole/ 50 Other 59 Trime-thoprim Epidermal Necrolysis Leukoencephalopathy Vancomycin 18 Allopurinol 43 Fingolimod 20 Ciprofloxacin 15 Omeprazole 16 Fluconazole 12 Mycophenolate 10 Ibuprofen 39 Clindamycin 10 Acetaminophen 39 Analgesics 91 Diclofenac 13 E ric Cropp, an Ohio hospital pharmacist involved in a tragic medication error, staff at the Death Due to Pharmacy Compounding Error Institute for Safe Medication Practices (ISMP) have been deeply saddened and greatly Reinforces Need for Safety Focus troubled to learn that he received 6 months in jail, 6 months home confinement with an An Injustice Has electronic sensor locked to his ankle after his Problem: As part of an ongoing collaboration with a provincial death investigation service, our sister organization, ISMP Canada, received a report regarding the death of a child who had ingested a Been Done: Jail release, 3 years probation, 400 hours of prescribed, compounded oral liquid suspension that contained the wrong medication. Time Given to community service, a fine of $5,000, and • Conclusion: The selection error described above, with its • Case Description: For about 18 months, a young child had been receiving a 3 gram dose of tryptophan 150 tragic result, could have occurred in any community or payment of court costs. Eric made a human Pharmacist Who mg/mL suspension (20 mL) by mouth at bedtime to treat hospital pharmacy or drug preparation facility that error that could have been made by others in a complex sleep disorder. Tryptophan was available as a compounds medications. Compounding of medications is a Made an Error dietary supplement in capsule form, but for this child, it healthcare given the inherent weaknesses in high-risk activity that results in a final product for which needed to be compounded in an appropriate dosage (ISMP , 2009) ingredients cannot be verified through physical form, as an oral suspension. A refill of the tryptophan our manual checking systems: he failed to examination. Before compounding is undertaken, prescription was ordered and picked up from the commercially available alternatives should be used if recognize that a pharmacy technician he was compounding pharmacy that had prepared the suspension in the past. That night, the child was given the usual available, and there should be an evidence-based or supervising had made a chemotherapy solution dose of medication; the next morning, the child was otherwise appropriate clinical rationale for the use of the found lifeless in bed. Post-mortem toxicology identified with far too much sodium chloride in it. The compounded product. lethal levels of the antispasticity agent baclofen, which final solution was supposed to contain 0.9% had not been prescribed for the child. sodium chloride but it was over 20%. 2
Recommend
More recommend