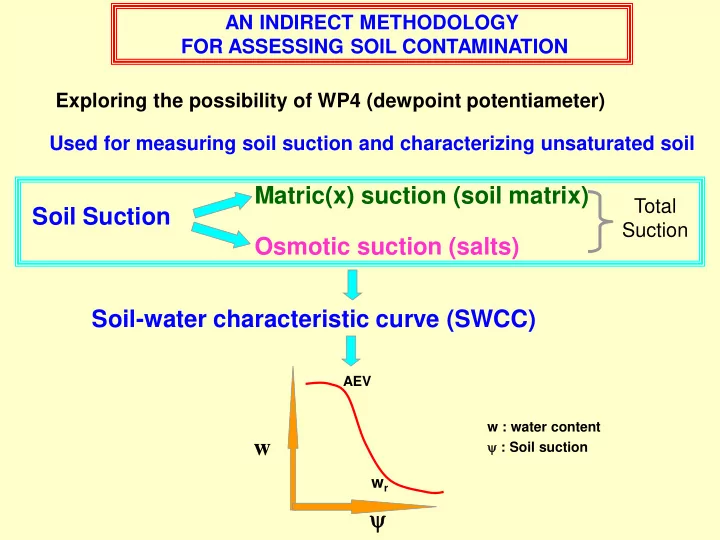

AN INDIRECT METHODOLOGY FOR ASSESSING SOIL CONTAMINATION Exploring the possibility of WP4 (dewpoint potentiameter) Used for measuring soil suction and characterizing unsaturated soil Matric(x) suction (soil matrix) Total Soil Suction Suction Osmotic suction (salts) Soil-water characteristic curve (SWCC) AEV w : water content : Soil suction w w r
Working principle of WP4 Block chamber Works on relative humidity principle Measuring range- 0 to 80 MPa WP4 measures total suction of soil Uncontaminated soil : Total suction = Matric(x) suction Contaminated soil : Total suction = Matric(x) suction + Osmotic suction SWCC of uncontaminated and contaminated soil of same type would be different The difference between SWCCs would indicate soil contamination
A Case study Soil used: Marine soil designated as contaminated soil (CS) Source: Collected from the coastal area of Mumbai, India Physical properties Chemical properties Soil property Value Specific gravity 2.64 Oxide % by weight Particle size characteristics SiO 2 33 Coarse sand (4.75-2.0 mm) 4 Al 2 O 3 11 Medium sand (2.0-0.420 mm) 9 Fe 2 O 3 12 Fine sand (0.420-0.074mm) 11 TiO 2 2 Silt size (0.074-0.002 mm) 44 CaO 6 Clay size (< 0.002 mm) 32 Chlorides (ppm) 9840 Consistency limits Sulphites (ppm) 40 Liquid limit (%) 61 CEC (meq/100g) 4.04 Plastic limit (%) 37 Plasticity index (%) 24 As such the soil is Soil Classification (USCS) MH contaminated
Soil subjected to washing to nullify contamination No. of washings LS Chloride (ppm) Sulphite (ppm) 1 2 6750 15 2 4 1850 10 3 6 800 10 4 8 250 5 5 10 90 < 5 100 1.0 Contaminated soil 90 Washed soil 0.9 80 0.8 70 0.7 60 (ms/cm) 0.6 50 w Difference due to 0.5 contamination 40 0.4 30 0.3 20 Washing nullifies contamination 0.2 10 0.1 0 0.0 0 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 10 10 10 10 10 10 10 10 No. of washings (kPa)
Corrosion Potential of Soils For geotechnical engineers, it’s very important subject Metal corrosion in undisturbed soils is generally very low regardless of the soil composition (e.g. metal piles, reinforcement of foundation etc.) Corrosion of metal (steel) in disturbed soils (e.g., buried pipelines that are backfilled) is strongly affected by soil conditions & properties. Soil changes its chemical and physical nature continuously over time and seasonally. Pipeline damage from pitting/corrosion
Soil Characteristics & Environmental Variables • Chloride content • Moisture content • Oxygen content/Redox potential • Soil permeability/texture • pH/Acidity • Temperature • Soil resistivity • Drainage characteristics • Sulfate/Sulfite ion concentrations • Microbiological activity • Stray currents (from cathodic protection, DC traction system viz., train, metro) • Spillage of corrosive substance/pollution
Soil Classification/Texture Clay in the soil mass reduces movement of air (oxygen) and water, i.e. low aeration, when wet, and hence increase in local (pitting) corrosion. High plasticity of clay (swelling/shrinking soils) can pull off susceptible coatings on the structures. Clay is susceptible to cracking (during wetting-drying cycles) which helps transport of air and moisture to the structures buried in it. Sand promotes aeration and moisture distribution & hence, soluble salts and gases (air/oxygen) are easily transported to structures, causing greater general corrosion but less pitting.
Bored Cast in-situ piles Chloride and Sulphate content of water found well within prescribed limit & hence water not corrosive. Ryzner index (RI) of water was found out to be 7.7 & hence water is corrosive and unsaturated Reinforcement in concrete pile exposed due to leaching of concrete
pH scale for Soils Ryznar Index Determines the degree of scale formation RI = 2 pH s – pH RI < 5.5 heavy scale will form 5.5 < RI < 6.2 scale will form 6.8 < RI < 8.5 water is corrosive Langelier Saturation Index (LI) RI > 8.5 water is very corrosive Determines if calcium carbonate will precipitate or not LI = pH – pH s pH = actual pH value measured in the water pH s = pH of the water in equilibrium with solid CaCO 3 If LI > 0 calcium carbonate will precipitate If LI < 0 calcium carbonate won’t precipitate The CaCO 3 layer deposited on surfaces acts as a protective coating.
ASSESSMENT OF CORROSION POTENTIAL OF SOILS Durability of underground structures is seriously affected by corrosion of the concrete (IS: 456-2000) Specifications for type of cement, minimum cement content, maximum water-cement ratio, etc., to be adopted stringently, based on the exposure of the concrete to different concentrations of sulphates in the soil or ground water. However, for assessment of corrosion potential of underground structures, chemical properties of the soil need to be considered in details. Corrosion is an electrochemical process Certain conditions must exist for the corrosion to occur ( corrosion cell ) Effects of soil characteristics on corrosion By Victor Chaker, J. David Palmer ASTM Committee G-1 on Corrosion of Metals
The “Corrosion cell” Metallic connection Soil Electrolyte Therefore properties of soils play a crucial role Electric current in accelerating corrosion. Properties of soils: Corrosion Electrical resistivity pH moisture content Porosity Electrochemical sulphate and chlorides content reaction redox potential presence of micro-organism temperature Cathode Anode are important for evaluating the corrosion Soil (Electrolyte) potential of soils (DIN 50929-3). For corrosion, the elements that are soluble in water are important: – Base forming: Na, K, Ca, Mg (raise pH). – Acid forming: Carbonate, Bicarbonate, Chloride ion, Nitrate, and Sulfate (lower pH).
Based on different soil characteristics, a certain rating (R1 to R6) for the soils has been assigned and the sum of these ratings is a measure of the overall soil corrosivity. Rating based on the soil fraction Rating based on the electrical resistivity Rating based on the pH Rating Based on the ground water status Rating based on the sulphite content Rating based on the chloride content
Rating based on the soil fraction Rating based on the electrical resistivity Resistivity ( .m) % by R2 Soil fraction R1 weight >500 +4 Clay & silt <10 +4 200 to 500 +2 10 to 30 +2 50 to 200 0 30 to 50 0 20 to 50 -2 50 to 80 -2 10 to 20 -4 >80 -4 <10 -6 Organic matter, e.g.: Higher conductivity: high corrosion rate muddy or swampy >5 -12 (efficient electrolyte) soils: peat, mud, marsh Severely polluted: Rating based on the pH due to fuel ash, slag - -12 coal, coke, refuse, PH R3 rubbish or waste water >9 +2 5.5 to 9 0 4.0 to 5.5 -1 <4 -3
Rating Based on the ground water status Rating based on the sulphite content Ground water status R4 Sulphite content (g/l) R5 No groundwater 0 <0.15 0 Groundwater -1 0.15 to 1 -2 Groundwater at times -2 1 to 2 -4 >2 -6 Rating based on the chloride content Total assessment of the corrosion potential Chloride content (ppm) R6 <100 0 Summation of R1- R6 Corrosion R potential 100-2000 -2 Virtually not 2000-10000 -4 0 corrosive >10000 -6 Slightly -1 to -4 corrosive Chloride ions: Cause pitting of steel -5 to -10 Corrosive and decrease soil resistivity. Highly < -10 corrosive
Soil Corrosivity based on Redox (Reduction-Oxidation) Potential ORP (Oxidation Reduction Potential) Dissolved Oxygen concentration in the soil moisture determines its RP(potential diff. between the electrodes), higher the oxygen content, higher would be the RP The difference in the RP may lead to the formation of the “corrosion cell” Low soil RP indicates conditions conducive to anaerobic microbiological activities. RP varies with time, moisture content variations, micro-organism activities etc. RP measurements may not be accurate assessment of corrosion potential of soils. In well aerated soils, Fe 3+ exhibits red, yellow, and brown colors. In poorly aerated soils, the oxygen content is low & soils are gray in color due to reduced state of the Fe. Redox Potential (mV) (Std. H Scale) Aeration Corrosivity >400 strong aeration Noncorrosive 200 to 400 Aeration Weak 100 to 200 weak aeration Moderate 0-100 Non to weak Severe Negative Not aerated Extremely sever
Recommend
More recommend