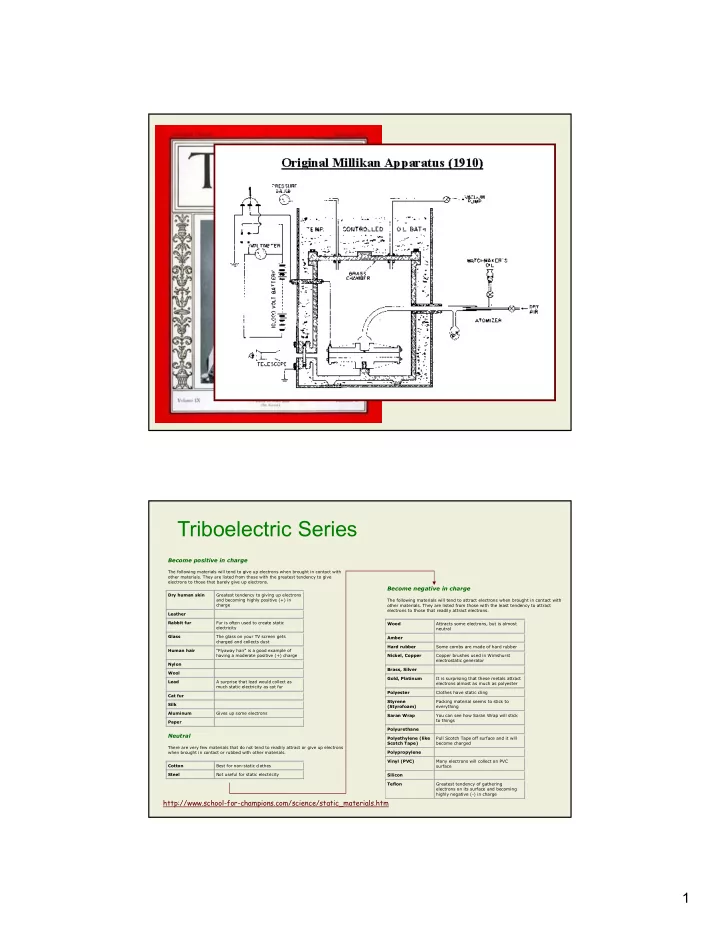

Triboelectric Series Become positive in charge The following materials will tend to give up electrons when brought in contact with other materials. They are listed from those with the greatest tendency to give electrons to those that barely give up electrons. Become negative in charge Dry human skin Greatest tendency to giving up electrons and becoming highly positive (+) in The following materials will tend to attract electrons when brought in contact with charge other materials. They are listed from those with the least tendency to attract electrons to those that readily attract electrons. Leather Rabbit fur Fur is often used to create static Wood Attracts some electrons, but is almost electricity neutral Glass The glass on your TV screen gets Amber charged and collects dust Hard rubber Some combs are made of hard rubber Human hair "Flyaway hair" is a good example of having a moderate positive (+) charge Nickel, Copper Copper brushes used in Wimshurst electrostatic generator Nylon Brass, Silver Wool Gold, Platinum It is surprising that these metals attract Lead A surprise that lead would collect as electrons almost as much as polyester much static electricity as cat fur Polyester Clothes have static cling Cat fur Styrene Packing material seems to stick to Silk (Styrofoam) everything Aluminum Gives up some electrons Saran Wrap You can see how Saran Wrap will stick to things Paper Polyurethane Neutral Polyethylene (like Pull Scotch Tape off surface and it will Scotch Tape) become charged There are very few materials that do not tend to readily attract or give up electrons when brought in contact or rubbed with other materials. Polypropylene Vinyl (PVC) Many electrons will collect on PVC Cotton Best for non-static clothes surface Steel Not useful for static electricity Silicon Teflon Greatest tendency of gathering electrons on its surface and becoming highly negative (-) in charge http://www.school-for-champions.com/science/static_materials.htm 1
An effort to reconstruct Millikan's "exemplary" experimental thinking revealed serious discrepancies between Millikan's notebooks and his published "raw" data (Holton, 1978). The numerous notes which are scattered across the pages cast further doubt on Millikan's integrity: This is almost exactly right & the best one I ever had!!! [20 December 1911] Exactly right [3 February 1912] Publish this Beautiful one [24 February 1912] Publish this surely / Beautiful !! [15 March 1912, #1] Error high will not use [15 March 1912, #2] Perfect Publish [11 April 1912] Won't work [16 April 1912, #2] Too high by 1½% [16 April 1912, #3] The notebooks reveal that, indeed, substantial data are missing from Millikan's published reports. Of 175 total drops documented in the notebooks, only 58 (barely one-third) appear in the final paper. By contrast, Millikan had announced in his 1913 paper that "It is to be remarked, too, that this is not a selected group of drops but represents all of the drops experimented on during 60 consecutive days, during which time the apparatus was taken down several times and set up anew" [his own emphasis!]. In his 1917 book, The Electron, he repeats this statement and then adds, "These drops represent all of those studied for 60 consecutive days, no single drop being omitted." http://www1.umn.edu/ships/ethics/millikan.htm 2
Was Millikan unethical? At first blush, this outrageous violation of scientific integrity would seem to discredit Millikan's findings. Even if one assumes that standards of reporting data earlier in the century were less rigorous, Millikan clearly misrepresented the extent of his data. One may caution students, however, that we may not want to conclude that therefore there was no good, "scientific" basis for his selective use of data . A more complete analysis of Millikan's notebooks, in fact, and of the nature of the experimental task that they crudely document, reveals more tellingly the reasons that Millikan included some drops and excluded others. http://www1.umn.edu/ships/ethics/millikan.htm 3
One may examine further specifically when the observations that Millikan excluded occurred. The first 68 observations, for instance, were omitted entirely. Why? Following February 13, 1912 (which marks the first published data), one may also note, the number of excluded results decreases as the series of experiments proceeds. Apparently, Millikan became more skilled as time went on at producing stable, reproducible data. Prior to February 13th, one may infer, he was still working the "bugs" out of the apparatus and gaining confidence in how to produce trustworthy results. That is, he was testing his equipment, not any theory of the electron or its charge. Here, the notebooks help focus our attention on the apparatus and the material conditions for producing evidence, not the role of the evidence itself. http://www1.umn.edu/ships/ethics/millikan.htm In fact, Franklin notes, Millikan threw out data that was "favorable" as well as "unfavorable" to his expectations. Clearly, Millikan's results were over-determined. That is, he had more data than he needed to be confident about his value for the electron's charge. Here, the redundancy of data was an implicit method for safeguarding against error. Thus, what appears as fraud from one perspective becomes, from an experimental perspective, a pattern of good technique. http://www1.umn.edu/ships/ethics/millikan.htm 4
There are rules for dealing with “bad” data: 1. Use median instead of mean for small sample sizes. 2. Acquire more data ($$). 3. Q test or Grubbs test to identify outliers (careful!). 4. Report the procedure used to deal with error. https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spect rpy/nmr/nmr1.htm 5
https://www.math10.com/en/algebra/probabilities/binomial- theorem/binomial-theorem.html 6
https://wonderopolis.org/wonder/what-is-pascals-triangle 45000 40000 35000 30000 25000 20000 15000 10000 5000 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 7
45000 40000 35000 30000 25000 20000 15000 10000 5000 0 1 2 3 4 5 6 7 8 9 1011121314151617 Defining a new dependent coordinate ( z ) in terms of x , the mean, and the standard deviation and plotting y versus z gives the graph on the right. (p. 70, Harris 8e) 8
https://www.chemistry.mcmaster.ca/esam/Chapter_3/section_2.html 9
10
11
N = number of measurements s = population standard s = standard deviation deviation 12
Three common cases for using the t test to compare measured values: 1. the mean of one sample is compared to a “known” value or established standard Example: molarity “known” to be 0.1147 M 2. the mean of one sample is compared to the mean of another sample Examples: concentration of phenol in Hogtown Creek; average height of CHM 3120 students 3. comparing individual data points in a set of paired measurements; this approach is commonly used to compare different analytical techniques (p. 78, Harris 8e) 13
Three common cases for using the t test to compare measured values: 1. the mean of one sample is compared to a “known” value or established standard Example: molarity “known” to be 0.1147 M 2. the mean of one sample is compared to the mean of another sample Examples: concentration of phenol in Hogtown Creek; average height of CHM 3120 students 3. comparing individual data points in a set of paired measurements; this approach is commonly used to compare different analytical techniques (p. 78, Harris 8e) 14
What is the pH of 1 10 8 M HNO 3 ( aq )? A. 6.0 B. 7.0 C. 8.0 D. 9.0 15
Rank the following solutions in order of increasing pH: 0.1 M HNO 2 , 0.1 M HNO 3 , 0.1 M NaNO 2 , 0.1 M NaNO 3 , 0.1 M NaOH A. HNO 2 < HNO 3 < NaNO 2 < NaNO 3 < NaOH B. HNO 3 < HNO 2 < NaNO 2 < NaNO 3 < NaOH C. HNO 2 < HNO 3 < NaNO 3 < NaNO 2 < NaOH D. HNO 3 < HNO 2 < NaNO 3 < NaNO 2 < NaOH E. HNO 3 < HNO 2 < NaOH < NaNO 3 < NaNO 2 16
14 12 10 8 pH HCl 6 HNO2 4 2 0 0 10 20 30 40 50 60 70 80 90 100 Volume of 0.10 M NaOH added (mL) 17
Which acid / conjugate base pair would be best to prepare a 6.85 pH buffer? Source: fac.ksu.edu.sa/sites/default/files/BUFFER_0.ppt Three practical methods to prepare a buffer: 1- First Method : By the Titration , in the presence of one of the two buffer forms with strong base or acid: Prepare a buffer composed of an acid and its salt by adding a strong base(e.g. NaOH) to a weak acid (e.g. Acetic acid) until the required pH is obtained If the other form of buffer is available (in this case sodium acetate), a strong acid is added (e.g. HCl) until the required pH is obtained. CH3COONa+HCl CH3COOH+NaCl So acetate buffer is formed(CH3COOH/CH3COONa) 18
Advantages: Easy to understand. Useful when only one form of the buffer is available (in this case acetic acid) Disadvantages: Slow. 1. May require lots of base (or acid). 2. 2- Second Method: Using the buffer pK a , calculate the amounts (in moles) of acid/salt or base/salt present in the buffer at the desired pH. If both forms (i.e., the acid and the salt) are available, convert the amount required from moles to grams ,using the molecular weight of that component, and then weigh out the correct amounts of both forms. Or convert moles to volume if the stock is available in the liquid form. 19
Recommend
More recommend