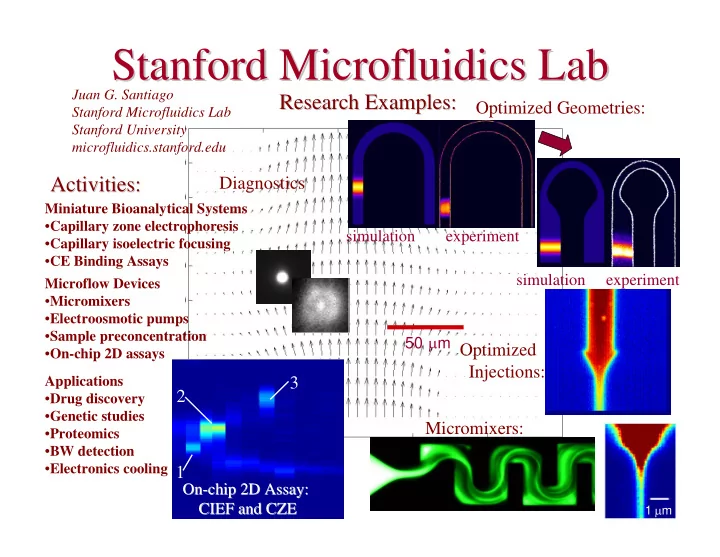

Stanford Microfluidics Microfluidics Lab Lab Stanford Juan G. Santiago Research Examples: Research Examples: Optimized Geometries: Stanford Microfluidics Lab Stanford University microfluidics.stanford.edu Activities: Diagnostics Activities: Miniature Bioanalytical Systems • Capillary zone electrophoresis simulation experiment • Capillary isoelectric focusing • CE Binding Assays simulation experiment Microflow Devices • Micromixers • Electroosmotic pumps • Sample preconcentration 50 µ m Optimized • On-chip 2D assays Injections: Applications 3 2 • Drug discovery • Genetic studies Micromixers: • Proteomics • BW detection • Electronics cooling 1 On- On -chip 2D Assay: chip 2D Assay: 1 µ m CIEF and CZE CIEF and CZE
Electrokinetics Microfluidics at Extreme Scales 10 µm 10 µm Juan G. Santiago Stanford Microfluidics Laboratory Mechanical Engineering Department Stanford University
Outline (time, concentration, length) • Introduction � Microfluidics � Electrokinetic (EK) flows • Electrokinetic instabilities (time) � Mechanism and model � Transition to chaos • ITP (concentration) � Sensitivity and practice � Extreme concentration scales • Nanochannel electrophoresis (length) � Small ion separations � DNA sample separation � Near-Future Work
Microfluidics • Applications � Point-of-care medical diagnostics � Bio-weapon detection � Pharmaceuticals/drug discovery � Environmental monitoring Image courtesy www.calipertech.com • Challenges and Advantages � Reduced reagent use � Specificity, robustness 0.7 cm � Portability vs. sensitivity � Integration and automation � Potential for parallel analyses www.nanogen.com
Processes in microfluidics ≠ ∇ > u u i u 0 drift ,1 drift ,2 drift Separation Stacking Hybridization Reaction
Separation in H-filter ∆ • diffusivity uFluidics ∆ • mobility On-chip CE ∆ • valence Nanochannel electrophoresis ≠ u u ∆ drift ,1 drift ,2 • affinity ∆ • polarizability ∇ ∆ • size/steric force
Electroosmotic flow Wall u(y) External Electric Field y x Wall y • Zeta vs. chemistry? λ D • EDL overlap? shear plane • Condensation? ζ ψ adsorbed ion
Electrohydrodynamics and Electrokinetics History Reuss, F.F. 1809. Memoires de ls Societe Imperial des Naturalistes de Moscow. 2:327. Gilbert, ~1580s Of the attraction exerted by amber
Electrokinetic Microfluidics 100 um λ d + + Pressure-driven Electrokinetic Glass or fused-silica microchannel wall + + + + + + + + + + + EOF - - + + - + - Electrophoresis + - + λ d Charge + + + + + λ d + double-layer + + + + + ++ + + + + Devasenathipathy S. and J. Santiago, Micro- Deprotonated silanol groups and nano-scale diagnostics , Springer-Verlag, 2003 • Electric control (no moving parts) • Switching, valving • Low dispersion • Integrated w/ separation techniques 100 um
Electrokinetic Instabilities
Complex electrokinetics • Sample preconcentration methods � Thermal gradient focusing � Field amplified sample stacking � Isotachophoresis 6 3 • On-chip two-dimensional assays CE dimension (mm) 5 2 1 B 4 Signal (au) 3 2 1 A C 1 D 0 IEF/EOF ∆ x = 4 W IEF dimension (mm) Herr, A.E. et al. Analytical Chemistry , Vol. 75, No. 5, pp. 1180-1187, 2003 • On-chip CE with unknown or poorly controlled sample chemistry • On-chip mixing and buffer exchange
Major Challenge in Heterogenous Particle visualization EK Systems: Instabilities Flows at Intersections: Axial interface: 1 mm 50 µ m Unstable, fluctuating concentrations in high- conductivity-gradient case 100 µ m 100 µ m
EK Instability Mechanism σ L σ L σ L Ε L σ L σ L σ L - - - σ H σ H +++ σ H σ H σ H σ H Ε H σ L Ε H Ε L σ L σ H σ L σ H σ L σ H σ H
Electrokinetic Flow Instabilities depth d • Generated by net charge in bulk δ σ L Ε L - - - Ε H ρ ≅ ε ⋅∇ σ σ ∆ / ~ E C +++ � E ε E E H σ H EV = L a U � Velocity scale: µ ε γ − 2 2 U d E d 1 ∗ ∗ ≡ = ∇ σ ev 0 � Controlling parameter: Ra � µ γ , e D D max ε γ − γδ ∆ − − − E 0 ( 1) / F 5 6 • C scales as ~ 10 10 0 • EOF coupling produces convective instab. • Multiple ion mobilities have severe impact Diffusion Electromigration ��� � ��� � � ����� χ σ 1 D ∑ ∑ = ∇ + ∇⋅ ∇ φ j 2 c ( c ) j j Oddy and Santiago, in press, Physics of Fluids , 2005. Dt Pe Pe j j j j Posner and Santiago, J. of Fluid Mechanics , pp. 1-42, 2006. Chen, C.-H. et al., J. of Fluid Mechanics , 524, pp. 263 – 303, 2005. Lin, H. et al., Vol. 16, No. 6 Physics of Fluids , p.1922-1935, 2004. 0
Electrokinetic instabilities t = 0.0 s t = 0.5 s t = 1.0 s t = 1.5 s t = 2.0 s t = 2.5 s t = 3.0 s t = 4.0 s t = 5.0 s Model Experiment Storey, B.D. et al. Physics of Fluids , Vol. 16, No. 6, p.1922-1935, 2004. Lin et al., submitted to J. Fluid Mechanics , 2005.
EKI in a cross intersection: Experiments Power spectra Ra e = 675 50 um Ra e = 800 Ra e = 2,000 Ra e = 2,700 f [1/sec]
Temporal Power Spectrum Ra e f [1/sec]
Correlation plots C1 Ra e =675 Ra e =800 C1 Ra e =2000 Ra e =2600 C2 C2
Isotachophoresis
ITP History • Kohlrausch : KRF function in 1897. • Tiselius : Moving boudnary electrophoresis, 1930 • Longsworth : Performed moving boundary electrophoresis in 1939. • Martin AJP: Displacement electrophoresis (also called ITP) in 1942 for cation. • Everaerts and Martin: First to perform ITP in thin capillaries (200 to 500 micron) in 1963. Used HEC to suppress EOF.
Detection Microchip/ Method Sample Electrolyte SE Comments Ref. Mode capillary didansyl-lysine Sodium tetraborate (0.5 First on-chip FASS FASS Fluore- Microchip 13.8 1995 mM and 500 mM) Run-time: 20 sec scence Carbonate (200 μ M and 32 Fluore- Microchip FITC-arginine 65 Six-channel geometry FASS 2001 mM) Run-time: 2 min scence Microchip Fluorescein sodium HEPES (0.1 mM and 100 100 Five-channel geometry. FASS Fluore- 2003 salt mM), NaCl (0.2 mM and Run-time: 1 min. scence 200 mM) Fluore- Microchip Fluorescein 175 mM Phosphate, DI 80 Narrow sample channel FASS 2003 disodium salt water Run-time: 2 min scence eTags LE: 25 mM imidazole, 20 Run-time: 1-2 min ITP Fluore- Microchip 530 2002 mM HCl, TE: 160 mM scence imidazole, 40 mM HEPES Microchip Fluorescein LE: 250 mM NaCl, TE: 95 500 Run-time: 2 min ITP Fluore- 2005 mM TAPS, 73 mM TEA scence PTH-aspartic acid, 100 mM MES and 100 mM First LVSS LVSS UV Capillary - 1992 PTH-glutamine acid histidine, DI water Run-time: 6-10 min L d) = 65 cm Dese, Amino 45 mM NaH 2 PO 4 and 15 Run-time: 4-6 min FASS UV Capillary 1000 1996 mM Na 2 HPO 4 , 60% v/v L = 24.6 cm 1 - propanol Maleic, fumaric 1 mM phosphoric acid, 40 Run-time: 5-10 min LVSS UV Capillary 300 1999 acids, bromide, mM potassium dihydrogen L = 61 cm nitrate phosphate 75 mM phosphate, DI water Run-time: 15-17 min FASI UV Capillary Bromide, nitrate, 1000 1999 bromate L = 25 cm 1 - naphthylamine, 50 mM phosphoric acid Run-time: 10-13 min FASI UV Capillary 200 2000 laudanosine with 20 % acetonitrile, DI L = 64 cm water NXX-066 LE: 10 mM NaOH titrated Highest ITP stacking ITP UV Capillary 5500 1998 with H 3 PO 4 , TE: 6.13 mM Run-time: 6-10 min L = 53.5 cm THeACl titrated with H 3 PO 4
Sensitivity in Capillary Electrophoresis (no stacking) 1.E-02 UV absorbance indirect fluorescence thermooptical end-column detection absorbance electrochemical detection 1.E-04 concentration (mol/l) mass spectrometric detection conductivity detection 1.E-06 UV absorbance with Z-shaped flow cells amperometric UV absorbance detection detection 1.E-08 Belder et al. electrochemical (2002) detection 1.E-10 Chen et al. radiochemical (1996) detection conductivity detection 1.E-12 with ITP stacking Ocvirk et al. fluorescence (1998) detection 1.E-14 1E-23 1E-21 1E-19 1E-17 1E-15 1E-13 1E-11 molar sensitivity (mol)
Single Interface Isotachophoresis E E Order of mobility Leading Ion (LE) ν > ν ν > Sample Ion • Characteristics Trailing Ion (TE) Sample zone grows with time. Counterion not shown � Stable concentration boundaries � Final Sample Concentration depends on Leading ion concentration �
ITP (S - ) Alexa fluor (1 µ M) HEPES (5mM)(TE) HEPES (5mM) NaCl (large excess) (LE) 50 µ m γ = 393 • Concentration enhancement greater than γ • Buffer selections allow for both ITP and FASS • ITP-type stacking with CE separation
Stable over 1000+ diameters Stable across flow geometries 50 µ m 5 mm Stability under large disturbances 50 µ m
Recommend
More recommend