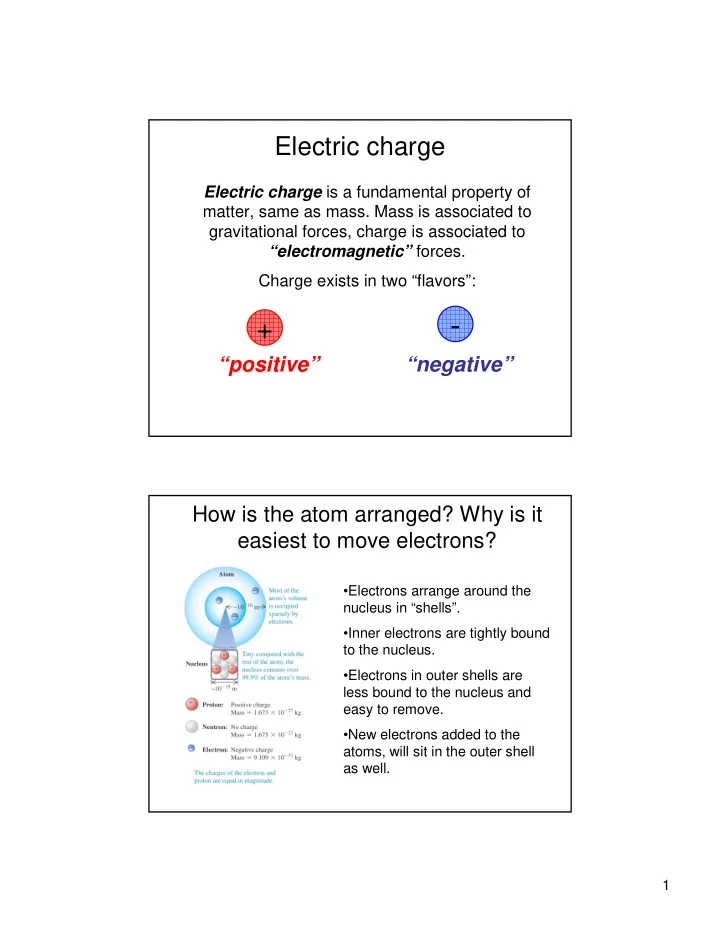

Electric charge Electric charge is a fundamental property of matter, same as mass. Mass is associated to gravitational forces, charge is associated to “electromagnetic” forces. Charge exists in two “flavors”: - + “positive” “negative” How is the atom arranged? Why is it easiest to move electrons? •Electrons arrange around the nucleus in “shells”. •Inner electrons are tightly bound to the nucleus. •Electrons in outer shells are less bound to the nucleus and easy to remove. •New electrons added to the atoms, will sit in the outer shell as well. 1
Lithium as a cation, an anion, and a neutral atom • Let’s study the subatomic arrangement of lithium with all charges balanced and the way only electrons move to make the atom an ion (+ or −). Charge is “quantized” Charge can only exist in “packets” formed by an integer number of “elementary or fundamental charges”, the charge of the electron (or proton) 1 . 60217653 10 − 19 C = × e This number is so small, that the charge can be considered to vary continuously in practice The unit of charge is the “Coulomb” Charge is conserved 2
Electric charge http://www.youtube.com/watch?v=pJ36EtABLAk&feature=related http://www.youtube.com/watch?v=Zo6I6rvtu2g&feature=related http://www.youtube.com/watch?v=F6v8wm7_vdQ Electric charge Glass rods, plastic tubes, silk, and fur can be used to demonstrate the movement of electrons and how their presence or absence make for powerful forces of attraction and repulsion. 3
Charging by conduction • Materials that allow easy passage of charge are called conductors . • Materials that resist electronic flow are called insulators . • The motion of electrons through conducts and about insulators allows us to observe “opposite charges attract” and “like charges repel.” http://www.youtube.com/watch?v=Dz_vvw_fsTo&feature=related Electrons move freely and charges may be induced 4
Polarizing an insulator • The motion of static charges about a plastic comb and light bits of paper can cause attractive forces strong enough to overcome the weight of the paper. http://www.youtube.com/watch?v=VhWQ-r1LYXY&NR=1 Charles Coulomb determined the electrostatic force law 5
Coulomb’s law 1 | | q q = 1 2 F 4 2 πε r 0 •The force on the particles has the same magnitude but opposite direction •They can be attractive (charges with opposite sign), or repulsive (charges with equal sign) •It is directly proportional to the charges, and inversely proportional to the square of the inter-charge distance •It does not depend on the mass!!! Coulomb’s law 1 | | q q = 1 2 F 4 2 πε r 0 8 . 854 10 − 12 C 2 /Nm 2 ε = × 0 1 9 2 2 = 9 . 0 × 10 Nm / C 4 πε 0 If the charges are 1C and the distance 1m, the force would be 9x10 9 N !!! This is about 1 million tons!!! Typical values of charge are in micro/nano-C 6
Electric vs. gravitational force Let us assume two point particles (alpha particles =nucleus of helium atom) with mass m=6.64x10 -27 kg and charge q=3.2x10 -19 C 1 2 2 q m = F g = F e G 2 4 2 πε r r 0 1 2 F q 3 . 1 10 35 = = × e 2 4 πε F G m 0 g Remember: Gravity is always attractive!!! Superposition of forces (I) We consider the charges as point-size objects q q n 0 i = = 0 F F i Total on on q q q ∑ ∑ 2 4 0 i 0 πε r i 0 i 0 i 7
Superposition of forces (II) 3 2 1 + + - d 2d F r F r 2 3 1 1 | | q q | | F = 1 3 1 4 πε ( 2 ) 2 d 0 | | 4 | | → F = F 1 | | q q 2 1 | | = F 2 3 2 2 4 πε d 0 Superposition of forces (III) 1 F r + 2 + F r F r 1 2 α 3 α + 3 F r 1 + 2 cos( ) = α F F 1 | | q q 1 1 x = πε 1 3 F 1 sin( ) 4 ( 2 2 ) = − α + F F x y 1 1 0 y 8
Recommend
More recommend