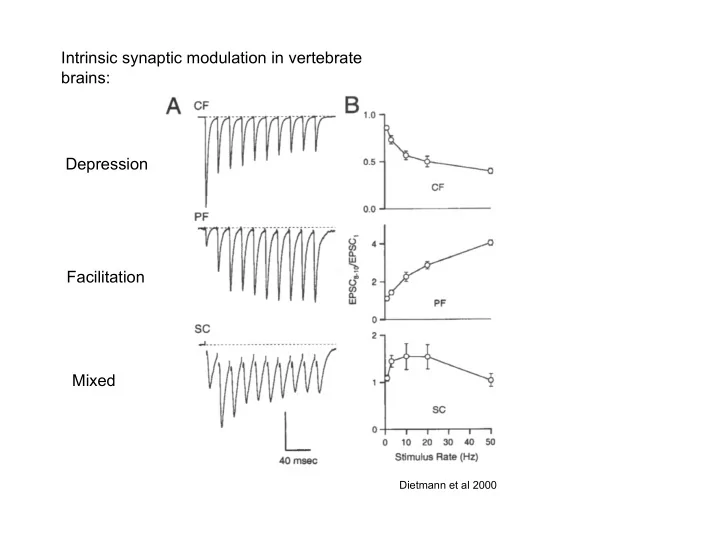

Intrinsic synaptic modulation in vertebrate brains: Depression Facilitation Mixed Dietmann et al 2000
Sites of Possible Change for Plasticity �
Facilitation: Minis same size, increase in frequency Residual Ca hypothesis for facilitation: Presynaptic Ca builds up with each AP
Sequestration of calcium after an action potential Uptake into mitochondria and E.R. Calcium ATPase Pump Na: Ca Exchange Calcium binding proteins Takes 100-500 msec to bring calcium levels to normal after an A.P.
Non-linear dependence of transmitter release on [Ca] i Ca 4 AP brings in 5 units of Ca 5 4 = 525 80% uptake before Next AP 1 unit left= 1 4 = 1 Next AP = 1 + 5 units 6 4 = 1296 (twice as much NT release!)
Facilitation BUT: 1) Realistic simulations of expected peak and residual Ca 2+ levels not able to account for facilitation. 2) Using Ca 2+ sensitive dyes, pre- synaptic [Ca 2+ ] does not account for enhanced synaptic transmission. 3) The time course of I K(Ca) is too fast. The decay of this current should reflect the decay of residual Ca 2+ . Ca++ may be acting at multiple sites of the synaptic machinery
PTP PTP: � 1) Correlates with decay of Ca 2+ image in whole terminal, not just at release sites;reduced by Ca 2+ chelators. � 2) In crustacean motor neurons, Na + has a role. Entry of Na + during AP firing may reduce the efficiency of the Na/Ca exchanger to get rid of Ca 2+ . � 3) Ca 2 + unloading from mitochrondria. � 3) In Aplysia- MEPP frequency up with PTP, but not amplitude. Both pre- and post- synaptic Ca 2+ chelators and postsynaptic hyperpolarization reduce PTP. �
Intrinsic synaptic modulation: � � Depression �
Shibere , a temperature sensitive fly mutant, vesicle recycling is blocked Depression correlates with depleted vesicles
Other mechanisms for depression: 1) Inactivation of I Ca during repetitive activity 2) Activation of inhibitory currents (IK Ca and ICl Ca 3) Transmitter release controlled by autoreceptors GABA responses are blocked by antagonists of presynaptic GABAa receptors. 4) Desensitization of post-synaptic receptors.
Overlapping Stages of Intrinsic Plasticity
Neuromodulatory control of synaptic strength Neuromodulation: Changing a neuron’s electrical properties through intracellular biochemical pathways initiated by neuroactive chemicals. Not usually rapid, not point to point, not simple excitation or inhibition
Neuromodulation: Extrinsic Synaptic Modulation Sites of Synaptic Change? � Control
Neuromodulation enhancing transmitter release � through AP broadening �
Transmitter release can be also strengthened without increased Ca entry
Modulators can have direct action on synaptic machinery. They can also affect other currents which control synaptic transmission: I K(Ca) , I Cl(Ca) Also possible post-synaptic actions
Brain neuromodulatory pathways
Brain neuromodulatory pathways- Serotonin imbalance
Electrical Synaptic Transmission Functions of electrical coupling: Fast Reliable Organize synchronous neuronal activity, Pass metabolites, second messengers, Ca2+ waves, Glial function Myelin organization.
Electrical synaptic transmission Dyes can pass between electrically coupled neurons
Elektrische Synapse (leech neurons) Electrical synaptic potential Electrical synaptic potential
Electrical synapse (gap junction)
Chemical Electrical Synapse structure discontinuity continuity Directionality Uni (?) Bi (?) Speed moderate (1-1.5 ms) FAST (0.1 ms) (escape networks) Threshold high none PSP shape transmitter removal same as pre
Recommend
More recommend