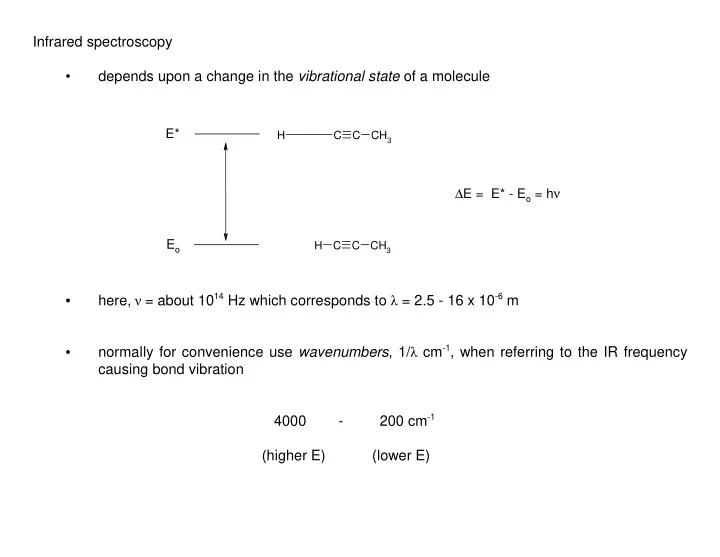

Infrared spectroscopy depends upon a change in the vibrational state of a molecule • E* H C C CH 3 ∆ E = E* - E o = h ν E o H C C CH 3 here, ν = about 10 14 Hz which corresponds to λ = 2.5 - 16 x 10 -6 m • normally for convenience use wavenumbers , 1/ λ cm -1 , when referring to the IR frequency • causing bond vibration 4000 - 200 cm -1 (higher E) (lower E)
The vibrational behavior of the chemical bonds in molecules is well-described by Hooke’s Law ν = ( / π µ 1 2 c k ) / so the frequency of the vibration depends upon the masses of the bonded atoms: the greater the difference, the lower the frequency • the strength of the bond: the weaker the bond, the lower the frequency • a change in the dipole moment of the bond as it vibrates •
How an IR spectrometer works detector source sample h ν (a heater) (film sandwiched (thermometer) between salt plates) • as the wavelength of radiation is varied, is the detector hot or not?
X X X ν X h ν X X 100 = cold 0 = hot absorbance
Recommend
More recommend