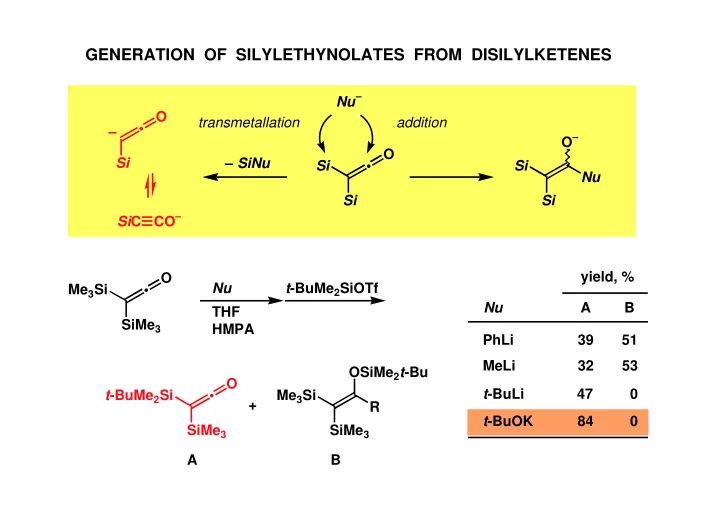

GENERATION OF SILYLETHYNOLATES FROM DISILYLKETENES Nu – O transmetallation addition • – O – O Si – SiNu Si • Si Nu Si Si CO – Si C yield, % O t -BuMe 2 SiOTf Nu Me 3 Si • A B Nu THF SiMe 3 HMPA PhLi 39 51 MeLi 32 53 OSiMe 2 t -Bu O t -BuLi 47 0 t -BuMe 2 Si • Me 3 Si + R t -BuOK 84 0 SiMe 3 SiMe 3 A B
Pd–MEDIATED KETENYLATION USING SILYLETHYNOLATE O O KO t -Bu K • Me 3 Si • ( i -Pr) 3 SiC COK THF Si( i -Pr) 3 Si( i -Pr) 3 HMPA O [( η 3 -C 3 H 5 )PdCl] 2 • THF Si( i -Pr) 3 –30 ˚C trace O • Cl –30 ˚C Si( i -Pr) 3 0%
Pd YNOLATE INTERMEDIATE Cl SiC CO K Pd 2 – KCl O • PdL n (OC ≡ CSi) –Pd(0)L n Si – SiY Y SiC CO Si Pd L n O • Si + Pd(0)L n Y Si
Pd–CATALYZED ALLYLATION OF DISILYLKETENES O O Pd(PPh 3 ) 4 • Me 3 Si • + Y THF Si( i -Pr) 3 Si( i -Pr) 3 rt, 12 h ketene:allyl compound:Pd = 20:20:1 yield, % Y O • Si OAc 75 Si OCO 2 CH 3 77 Pd(OC CSi) Pd OR Cl 0 Si OR
REACTION OF VARIOUS SILYLKETENES O O Pd(PPh 3 ) 4 R 1 • • + OAc R 2 R 2 THF rt, 12 h ketene:allyl acetate:Pd = 20:20:1 R 1 R 2 yield, % SiMe 3 Si( i -Pr) 3 75 SiMe 3 SiMe 2 t -Bu 56 SiMe 3 SiMe 3 60 63 a SiMe 3 SiMe 3 SiMe 3 n -Bu 0 a Ketene:allyl acetate:Pd = 20:40:1.
VARIOUS ALLYL ACETATES R 2 R 4 R 2 R 4 O O Pd(PPh 3 ) 4 Me 3 Si • • + R 1 R 1 OAc THF R 3 Si( i -Pr) 3 Si( i -Pr) 3 R 3 rt, 5–12 h ketene:allyl acetate:Pd = 5:5:1 a Side reaction products alyll acetate product yield, % O R 1 R 2 R 3 R 4 R 1 R 2 R 3 R 4 H • H H Me H H Me 67 H H Si( i -Pr) 3 31 a H H Me H H Me H H R 0 a H H Me Me H Me Me H H H 57 Ph H H Ph H H H H H Ph Ph H H H 42 (R = H, Me)
A POSSIBLE CATALYTIC CYCLE O PdL n • Y Si Y PdL n (OC ≡ CSi) Pd L n O • Si SiY Si
ALLYLIC SUBSTITUTION WITH ORGANOSILICON COMPOUNDS Pd Nu + R 3 Si–Y R 3 Si –Nu + Y R 1 R 1 : Y OAc OCO 2 CH 3 OCOCF 3 O O : CN SiR 3 Nu R 2 R 2 R 1 O R 1 J. Tsuji et al. , Chem. Lett. , 1983 , 1325. J. Tsuji et al. , Tetrahedron Lett., 1984 , 25 , 4783. Y. Tsuji et al. , J. Org. Chem., 1996 , 61 , 5779. Y. Tsuji et al. , Organometallics., 1998 , 17 , 4835.
A PRECEDENT FOR C–C BOND FORMATION USING SILYLETHYNOLATE Me 3 Si N 2 + CO Me 3 SiC COLi –78 ˚C Li 1 atm SiMe 3 O H + AlMe 3 O –78 → 20 ˚C O –78 → 0 ˚C 93% S. Murai et al. , J. Am. Chem. Soc., 1996 , 118 , 7634.
SUMMARY O O Pd(PPh 3 ) 4 Me 3 Si • • + Y THF R' R' SiR 3 SiR 3 up to 77% PdL n (OC ≡ CSi) R' R 3 Si : Me 3 Si, t -BuMe 2 Si, ( i -Pr) 3 Si R' : H, Me, Ph Y : OAc, OCO 2 CH 3
Recommend
More recommend