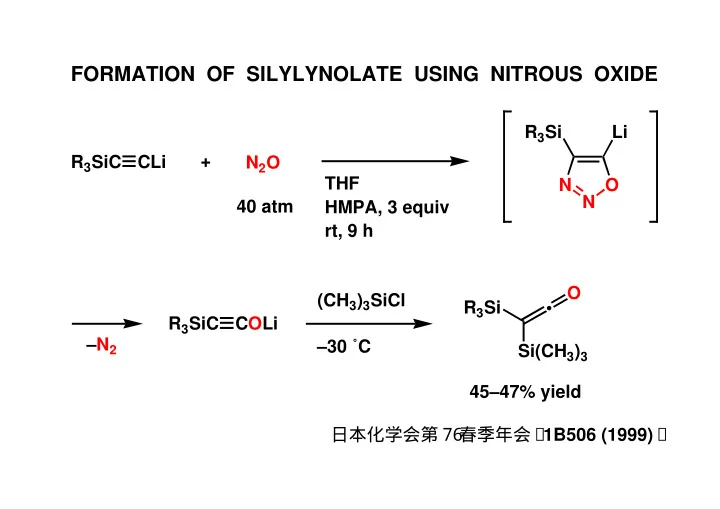

FORMATION OF SILYLYNOLATE USING NITROUS OXIDE R 3 Si Li + R 3 SiC CLi N 2 O THF N O N 40 atm HMPA, 3 equiv rt, 9 h O (CH 3 ) 3 SiCl R 3 Si • R 3 SiC COLi –N 2 –30 ˚C Si(CH 3 ) 3 45–47% yield 日本化学会第7 6 春季年会, 1B506 (1999) .
Al(CH 3 ) 3 –PROMOTED KETENYLATION USING SILYLYNOLATE (CH 3 ) 3 Si + (CH 3 ) 3 SiC COLi N 2 CO –78 ˚C Li 1 atm Si(CH 3 ) 3 O H + Al(CH 3 ) 3 O O –78 ˚C – 0 ˚C –78 ˚C – 20 ˚C 93% S. Murai et al. , J. Am. Chem. Soc., 1996 , 118 , 7634.
Pd–PROMOTED KETENYLATION USING SILYLYNOLATE ( i -C 3 H 7 ) 3 SiC CLi + N 2 O ( i -C 3 H 7 ) 3 SiC COLi THF–ether 40 atm HMPA, 3 equiv rt, 9 h O [( η 3 -C 3 H 5 )PdCl] 2 • THF Si( i -C 3 H 7 ) 3 –30 ˚C trace O Cl • –30 ˚C Si( i -C 3 H 7 ) 3 0%
Pd YNOLATE INTERMEDIATE Cl SiC CO Li Pd 2 – LiCl O • CSi] – PdL n [OC –Pd(0)L n SiR 3 – SiY Y SiC CO Si Pd L n O • Si + Pd(0)L n Y SiR 3
KETENYLATION OF ALLYL ACETATES O O Pd[P(C 6 H 5 ) 3 ] 4 O (CH 3 ) 3 Si • R • + R THF O Si( i -C 3 H 7 ) 3 Si( i -C 3 H 7 ) 3 rt, 5–12 h ketene:allyl compound:Pd = 5:5:1 yield, % R R yield, % CH 2 CH 2 75 31 CH 2 0 67 CH 2 O R ( i -C 3 H 7 ) 3 Si • R = H, CH 3 H
REGIOSELECTIVITY FOR THE KETENYLATION PdL n PdL n OCOCH 3 OCOCH 3 Pd L n OCOCH 3 O (CH 3 ) 3 Si • Si( i -C 3 H 7 ) 3 O O • • Si( i -C 3 H 7 ) 3 Si( i -C 3 H 7 ) 3
REACTION OF VARIOUS SILYLKETENES O O Pd[P(C 6 H 5 ) 3 ] 4 R 1 • • + OCOCH 3 R 2 R 2 THF rt, 12 h ketene:allyl acetate:Pd = 20:20:1 R 1 R 2 yield, % Si(CH 3 ) 3 Si( i -C 3 H 7 ) 3 75 Si(CH 3 ) 3 Si(CH 3 ) 2 ( t -C 4 H 9 ) 56 Si(CH 3 ) 3 Si(CH 3 ) 3 60 63 a Si(CH 3 ) 3 Si(CH 3 ) 3 Si( i -C 3 H 7 ) 3 CH 2 CH CH 2 0 a Ketene:allyl acetate:Pd = 20:40:1.
KETENYLATION OF VARIOUS ALLYL COMPOUNDS O O Pd[P(C 6 H 5 ) 3 ] 4 • (CH 3 ) 3 Si • + Y THF Si( i -C 3 H 7 ) 3 Si( i -C 3 H 7 ) 3 rt, 12 h ketene:allyl compound:Pd = 20:20:1 yield, % Y O Si • OCOCH 3 75 Si OCO 2 CH 3 77 Pd[OC CSi] Pd OR Cl 0 Si OR
ALLYLIC SUBSTITUTION WITH ORGANOSILICON COMPOUNDS R 1 Pd + Nu R 3 Si –Nu R 3 Si–OY + OY R 1 = OY OCOCH 3 OCO 2 CH 3 OCO 2 CF 3 O O = SiR 3 CN Nu R 2 R 2 R 1 R 1 O J. Tsuji et al. , Chem. Lett. , 1983 , 1325. J. Tsuji et al. , Tetrahedron Lett., 1984 , 25 , 4783. Y. Tsuji et al. , J. Org. Chem., 1996 , 61 , 5779. Y. Tsuji et al. , Organometallics., 1998 , 17 , 4835.
A POSSIBLE CATALYTIC CYCLE O PdL n • Y Si Y Pd PdL n [OC CSi] L n O • Si SiY Si
SUMMARY O O Pd[P(C 6 H 5 ) 3 ] 4 (CH 3 ) 3 Si • • + Y THF R' R' SiR 3 SiR 3 up to 77% PdL n [OC CSiR 3 ] R' R 3 Si = (CH 3 ) 3 Si, ( t -C 4 H 9 )(CH 3 ) 2 Si, ( i -C 3 H 7 ) 3 Si R' = H, CH 3 , C 6 H 5 , CO 2 C 2 H 5 Y = OCOCH 3 , OCO 2 CH 3
Recommend
More recommend