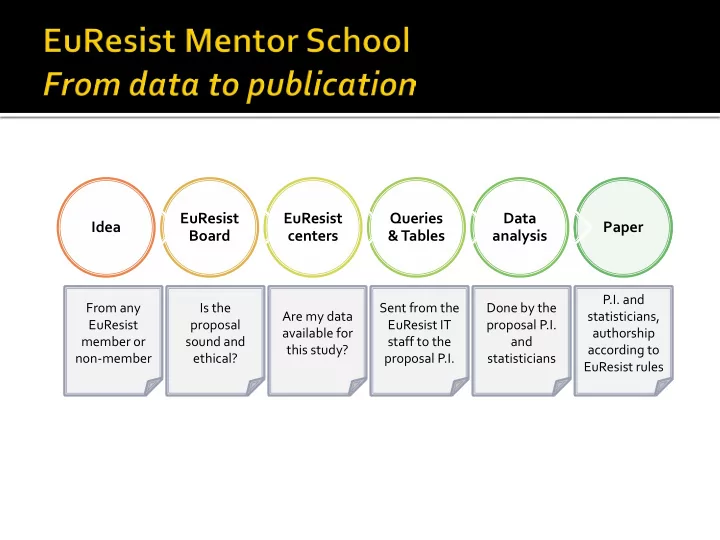

EuResist EuResist Queries Data Idea Paper Board centers & Tables analysis P.I. and From any Is the Sent from the Done by the Are my data statisticians, EuResist proposal EuResist IT proposal P.I. available for authorship member or sound and staff to the and this study? according to non-member ethical? proposal P.I. statisticians EuResist rules
EuResist EuResist Queries Data Idea Paper Board centers & Tables analysis P.I. and From any Is the Sent from the Done by the Are my data statisticians, EuResist proposal EuResist IT proposal P.I. available for authorship member or sound and staff to the and this study? according to non-member ethical? proposal P.I. statisticians EuResist rules Contribution of each individual step to the overall effort and time required
IT & data Biomedical management domain domain
IT & data Biomedical management domain domain Learn the basics of data Learn the basics of the structure biomedical topic Avoid professional lingo Avoid professional lingo
IT & data Biomedical domain management domain Create and nurture a generation of "middleware" professionals Increase accuracy of data collection Minimize misunderstandings in generating datasets Expedite the process from data to product
Integration between the biomed and IT domains Cooperation & networking Periodical event Mostly hands-on format Working group with biomedical and IT tutor Queries are followed up remotely after the on- site course Suggestions for improvement always welcome!
09:00 – 09:30 Introduction and Signing NDAs Let’s work! (Group A: floor -1; Group B: ground floor) 09:30 – 13:00 Query 1: Does resistance to NRTIs influence response to INSTI plus NRTIs? 13:00 – 14:00 Lunch 14:00 – 17:30 Query 2: Efficacy of dual regimens as switch in patients with undetectable VL, including the influence of past resistance 17:30 – 18:30 Presentation of the work done (plenary)
2014 – Major system revision to include Hepatitis data and 2005 – ARCA turn to complete opened to research Electronic Medical proposals Recording system 2004 – ARCA web site and db launched 2002 – Collection of clinical data started to complement virological data
Building treatment response models and treatment decision tools On-line Electronic Medical Recording system HIV, HBV, HCV genotype interpretation via the AntiViroScan algorithm, curated by the University of Siena Repository for HIV, HBV, HCV research proposals requiring a large amount of data or a specific uncommon patient population Middle layer between local clinics and national/international public programs , particularly focusing on response to antiviral therapy or molecular epidemiology www.dbarca.net → Obiettivi
www.dbarca.net → Interroga il db in tempo reale
P.I. plus up to 4 collaborators Up to 15 total authors adding the ARCA Authorship Delegates (subject to editorial policy limits) Included in authorship Not included in authorship but credits saved for future studies Study dataset Individual DB’s (web site -> Statuto e regolamenti) www.dbarca.net → Statuto e regolamenti
Publication system launched in 2005 (HIV) 110 data requests including 18 from abroad Only 4 not approved 107 publications 78 Conference papers 36 Papers Low paper/conference ratio (0.5) Publications (public area) and requests (private area) tracking available on the web site (web site -> Pubblicazioni; web site -> Richieste) www.dbarca.net → Pubblicazioni
Use of real life data instead of performing prospective clinical studies Introduction to the data base structure Introduction to structured query language Workshop queries: Query 1: Does resistance to NRTIs influence response to INSTI plus NRTIs? Query 2: Efficacy of dual regimens as switch in patients with undetectable VL, including the influence of past resistance
Follow-up All participants will be contacted to proceed with the analysis with the aim to reach a publication with all active participants Who is interested to work on the db? Who would like to participate in this workshop next year (2 groups: advanced and basic…..)
Cooperation of partners (idea by ARCA) Each partner remains owner of his data Submit data to an integrated database Use data from the EuResist database Agree that others use your data upon request (case by case decision)
Recommend
More recommend