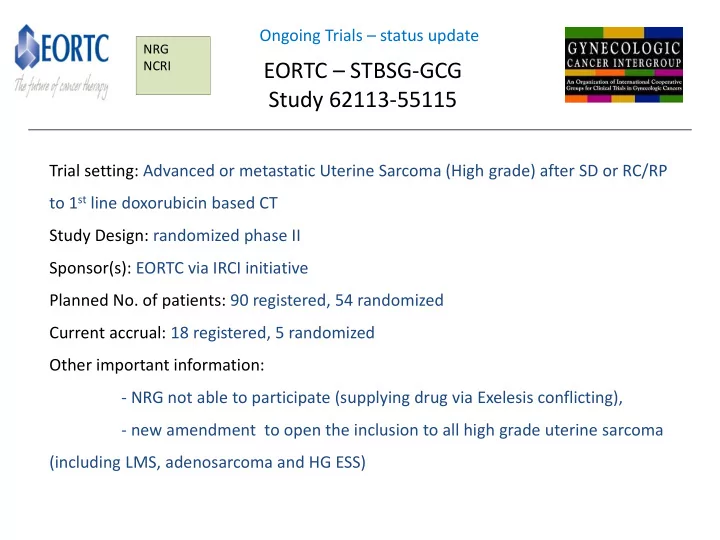

Ongoing Trials – status update NRG NCRI EORTC – STBSG-GCG Study 62113-55115 Trial setting: Advanced or metastatic Uterine Sarcoma (High grade) after SD or RC/RP to 1 st line doxorubicin based CT Study Design: randomized phase II Sponsor(s): EORTC via IRCI initiative Planned No. of patients: 90 registered, 54 randomized Current accrual: 18 registered, 5 randomized Other important information: - NRG not able to participate (supplying drug via Exelesis conflicting), - new amendment to open the inclusion to all high grade uterine sarcoma (including LMS, adenosarcoma and HG ESS)
EORTC – STBSG-GCG Study 62113-55115: A randomized double-blind phase II study evaluating the role of maintenance therapy with cabozantinib in High Grade Uterine Sarcoma (HGUtS) after stabilization or response to doxorubicin +/- ifosfamide following surgery or in metastatic first line treatment SC: Nick Reed , NHS Greater Glasgow & Clyde, UK (GCG) SC: Isabelle Ray-Coquard , Centre Leon Berard, France (STBSG)
Study design
Main eligibility criteria • At registration: o Patients who are suitable for treatment with doxorubicin +/- ifosfamide and fall within one of the following patient populations: HGUS, HGESS, HGLMS and HG adenosarcoma • At randomization: o Central pathological confirmation: Histological evidence of HGUS, HGESS, HGLMS and HG adenosarcoma o Non-progressive patients (CR, PR, SD) after first line treatment (standard chemotherapy consisting of 4 to 6 cycles of doxorubicin alone or in combination with ifosfamide) and at time of randomization o The subject's organ, marrow function and laboratory values need to be within the defined ranges before randomization
Endpoints Primary endpoint • PFS rate at 4 months Secondary endpoints • PFS (RECIST 1.1) • OS • Response rate and duration of response to cabozantinib (RECIST 1.1) • Response rate to doxorubicin-based chemotherapy after registration • HRQoL (QLQ-C30 + QLQ-EN24) • Safety profile of maintenance therapy & at cross-over
Accrual: Registration
Accrual per institution (as of 11/05/2017) Institution # Registered # Randomized Centre Leon Berard (227) 10 3 Institut de Cancerologie de l’Ouest (ICO) - 3 0 Centre Rene Gauducheau (235) Academisch Medisch Centrum - Universiteit 1 1 van Amsterdam (342) Hospital Universitario San Carlos (366) 1 1 Cambridge University Hospital NHS - 1 0 Addenbrookes Hospital (632) Universitair Ziekenhuis Antwerpen (117) 1 0 Greater Glasgow and Clyde - Beatson West 1 0 of Scotland Cancer Centre (6982) Total 18 5 Other authorized institutions did not yet register a patient. Target accrual = 54 randomized patients
Accrual per group (as of 11/05/2017) Group # Registered # Randomized EORTC STBSG/GCG 16 5 NCRI 2 0 Total 18 5 Reason of screening failure # failures No central pathological confirmation of disease 4 Patient refusal 2 Inadequate organ or marrow function or lab values 1 Randomization outside allowed time window 1 Progression during first line treatment 2 Patient condition deteriorated during first line treatment 1 Total 11
Recommend
More recommend