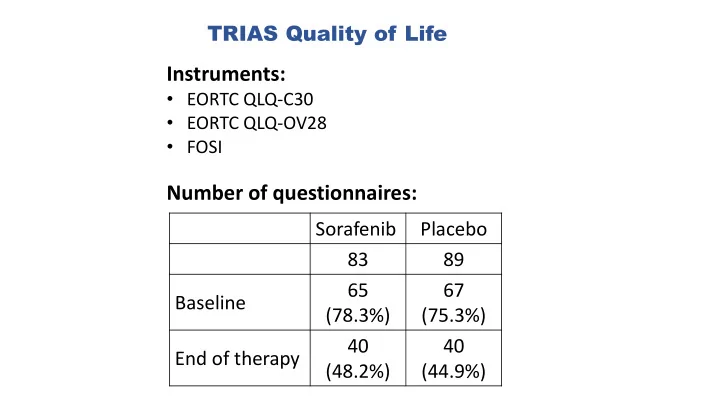

TRIAS Quality of Life Instruments: • EORTC QLQ-C30 • EORTC QLQ-OV28 • FOSI Number of questionnaires: Sorafenib Placebo 83 89 65 67 Baseline (78.3%) (75.3%) 40 40 End of therapy (48.2%) (44.9%)
TRIAS Quality of Life EORTC QLQ-C30 Baseline to end of therapy Changes in mean scores of global quality of life and functional scales Improvement Worsening Global QoL Physical Role Emotional Social -30 -25 -20 -15 -10 -5 0 5 10 15 ▬ 95% CI Sorafenib Placebo
TRIAS Quality of Life EORTC QLQ-C30 Baseline to end of therapy Changes in mean scores of symptom scales Improvement Worsening Fatigue Nausea/vomiting Dyspnea Appetite loss Constipation Diarrhea -20 -10 0 10 20 30 ▬ 95% CI Sorafenib Placebo
TRIAS Quality of Life EORTC QLQ-OV28 Baseline to end of therapy Changes in mean scores of symptom scales Improvement Worsening Abdominal/gastrointestinal Peripheral neuropathy Other chemotherapy- related side effects Hormonal Body image Attitude to disease and treatment -20 -10 0 10 20 30 ▬ 95% CI Sorafenib Placebo
TRIAS Quality of Life FOSI Baseline to end of therapy Changes in mean scores Improvement Worsening FOSI -20 -10 0 10 20 ▬ 95% CI Sorafenib Placebo ▬ 95%
TRIAS Quality of Life Summary Significant worsening from baseline to end of therapy: • Role functioning, dyspnea, constipation, and diarrhea with CID • Physical functioning, fatigue, nausea/vomiting, peripheral neuropathy, and body image without CID Significant difference between arms in change from baseline to end of therapy: • Attitude to disease and treatment (p=0.038) • Sorafenib arm improvement (8.2 points) and placebo arm worsening (1.7 points) Non-significant difference between arms in change from baseline to end of therapy: • Favoring Sorafenib: Global QoL (8.6 points), constipation (8.1 points) • Favoring placebo: Dyspnea (12.2 points), diarrhea (9.4 points) CID : Clinical important difference (>10 points)
Recommend
More recommend